Lipid Biomarkers From Microbial Mats on the McMurdo Ice Shelf, Antarctica: Signatures for Life in the Cryosphere
- PMID: 35756013
- PMCID: PMC9232131
- DOI: 10.3389/fmicb.2022.903621
Lipid Biomarkers From Microbial Mats on the McMurdo Ice Shelf, Antarctica: Signatures for Life in the Cryosphere
Abstract
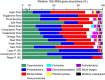
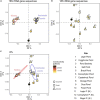
Persistent cold temperatures, a paucity of nutrients, freeze-thaw cycles, and the strongly seasonal light regime make Antarctica one of Earth's least hospitable surface environments for complex life. Cyanobacteria, however, are well-adapted to such conditions and are often the dominant primary producers in Antarctic inland water environments. In particular, the network of meltwater ponds on the 'dirty ice' of the McMurdo Ice Shelf is an ecosystem with extensive cyanobacteria-dominated microbial mat accumulations. This study investigated intact polar lipids (IPLs), heterocyte glycolipids (HGs), and bacteriohopanepolyols (BHPs) in combination with 16S and 18S rRNA gene diversity in microbial mats of twelve ponds in this unique polar ecosystem. To constrain the effects of nutrient availability, temperature and freeze-thaw cycles on the lipid membrane composition, lipids were compared to stromatolite-forming cyanobacterial mats from ice-covered lakes in the McMurdo Dry Valleys as well as from (sub)tropical regions and hot springs. The 16S rRNA gene compositions of the McMurdo Ice Shelf mats confirm the dominance of Cyanobacteria and Proteobacteria while the 18S rRNA gene composition indicates the presence of Ochrophyta, Chlorophyta, Ciliophora, and other microfauna. IPL analyses revealed a predominantly bacterial community in the meltwater ponds, with archaeal lipids being barely detectable. IPLs are dominated by glycolipids and phospholipids, followed by aminolipids. The high abundance of sugar-bound lipids accords with a predominance of cyanobacterial primary producers. The phosphate-limited samples from the (sub)tropical, hot spring, and Lake Vanda sites revealed a higher abundance of aminolipids compared to those of the nitrogen-limited meltwater ponds, affirming the direct affects that N and P availability have on IPL compositions. The high abundance of polyunsaturated IPLs in the Antarctic microbial mats suggests that these lipids provide an important mechanism to maintain membrane fluidity in cold environments. High abundances of HG keto-ols and HG keto-diols, produced by heterocytous cyanobacteria, further support these findings and reveal a unique distribution compared to those from warmer climates.
Keywords: Antarctica; bacteriohopanepolyol; cyanobacteria; heterocyte glycolipids; homeoviscous adaptation; intact polar lipid; microbial mats.
Copyright © 2022 Evans, Kalambokidis, Jungblut, Millar, Bauersachs, Grotheer, Mackey, Hawes and Summons.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Atkinson M. J. (1987). Low phosphorus sediments in a hypersaline marine bay. Estuar. Coast. Shelf Sci. 24 335–347.
-
- Bale N. J., Hopmans E. C., Dorhout D., Stal L. J., Grego M., van Bleijswijk J., et al. (2018). A novel heterocyst glycolipid detected in a pelagic N2-fixing cyanobacterium of the genus Calothrix. Org. Geochem. 123 44–47.
-
- Bauersachs T., Compaoré J., Hopmans E. C., Stal L. J., Schouten S., Sinninghe Damsté J. S. (2009a). Distribution of heterocyst glycolipids in cyanobacteria. Phytochemistry 70 2034–2039. - PubMed
LinkOut - more resources
Full Text Sources

