Long-Term Humoral Response After a Second Dose of SARS-CoV-2 mRNA Vaccine in Japanese Kidney Transplant Recipients
- PMID: 35756063
- PMCID: PMC9218893
- DOI: 10.3389/fmicb.2022.922042
Long-Term Humoral Response After a Second Dose of SARS-CoV-2 mRNA Vaccine in Japanese Kidney Transplant Recipients
Abstract
Background: The mortality rate due to COVID-19 in kidney transplant recipients (KTRs) is 16.8 to 32%. Vaccination against COVID-19 is expected to contribute to the prevention of infection, severe disease, and mortality; however, it has been reported that the humoral response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mRNA vaccine in KTRs is poor. Vaccination strategies against COVID-19 vary from country to country, and in Japan, the third dose is given 6 months after the second dose. Few studies have evaluated long-term humoral responses after the second dose of SARS-CoV-2 mRNA vaccine. In addition, the superiority of BNT162b2 vaccine and mRNA-1,273 vaccine in KTRs regarding humoral response is controversial.
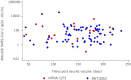
Methods: Ninety-four KTRs were administered a second dose of the BNT162b2 or mRNA-1,273 vaccines, and anti-spike (anti-S) and anti-nucleocapsid (anti-N) SARS-CoV-2 antibody levels were measured 5 months (149.2 ± 45.5 days) later. The cutoff value of anti-S antibodies was defined ≥50 AU/ml and 1.4 Index for anti-N antibodies. The primary outcome was the rate of seropositivity, and factors associated with an appropriate humoral response were assessed by univariate and multivariate analysis.
Results: Of 94 KTRs, only 45 (47.9%) patients were positive for anti-S antibodies. The median anti-S SARS-CoV-2 IgG antibody titers was 35.3 (Interquartile range 3.8 to 159.7). Anti-N SARS-CoV-2 IgG antibodies in all patients were < 1.4 Index. Response to SARS-CoV-2 mRNA vaccines were 43.2 and 65% for BNT162b2 and mRNA-1,273, respectively (p = 0.152). In comparison with high-dose, low-dose of mycophenolic acid was a robust factor associated with an adequate humoral response.
Conclusion: The long-term humoral response after a second dose of SARS-CoV-2 mRNA vaccine in Japanese KTRs was poor. In comparison with high-dose, low-dose mycophenolic acid was related to an appropriate humoral response. Five months is too long to wait for a 3rd dose after 2nd dose of SARS-CoV-2 vaccine in KTRs. In this cohort, there was no statistical difference in humoral response to the BNT162b2 and mRNA-1,273 vaccines. Additional large observational studies and meta-analyses are needed to clarify the factors related to an appropriate humoral immune response to COVID-19 vaccination.
Keywords: COVID-19; SARS-CoV-2 vaccine; anti-S SARS-CoV-2 IgG antibody titers; humoral response; kidney transplant recipients.
Copyright © 2022 Ohki, Kawabe, Yamamoto, Katsumata, Nakada, Kobayashi, Urabe, Miki, Yamada, Kimura, Matsuo, Tanno, Horino, Ohkido, Yamamoto and Yokoo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Humoral and cellular immune response to SARS-CoV-2 mRNA BNT162b2 vaccine in pediatric kidney transplant recipients compared with dialysis patients and healthy children.Pediatr Nephrol. 2023 Jul;38(7):2199-2208. doi: 10.1007/s00467-022-05813-w. Epub 2022 Dec 2. Pediatr Nephrol. 2023. PMID: 36459243 Free PMC article.
-
Humoral and cellular immune response and the safety of third SARS-CoV-2 mRNA vaccine with longer interval after the second vaccination in kidney transplant recipients.Front Immunol. 2022 Nov 30;13:1050211. doi: 10.3389/fimmu.2022.1050211. eCollection 2022. Front Immunol. 2022. PMID: 36532067 Free PMC article.
-
Comparison of SARS-CoV-2 Antibody Response 4 Weeks After Homologous vs Heterologous Third Vaccine Dose in Kidney Transplant Recipients: A Randomized Clinical Trial.JAMA Intern Med. 2022 Feb 1;182(2):165-171. doi: 10.1001/jamainternmed.2021.7372. JAMA Intern Med. 2022. PMID: 34928302 Free PMC article. Clinical Trial.
-
Seroconversion rates in kidney transplant recipients following SARS-CoV-2 vaccination and its association with immunosuppressive agents: a systematic review and meta-analysis.Clin Exp Vaccine Res. 2023 Jan;12(1):13-24. doi: 10.7774/cevr.2023.12.1.13. Epub 2023 Jan 31. Clin Exp Vaccine Res. 2023. PMID: 36844682 Free PMC article. Review.
-
Immune Response to SARS-CoV-2 Vaccine among Heart Transplant Recipients: A Systematic Review.Clin Med Insights Circ Respir Pulm Med. 2022 Jun 5;16:11795484221105327. doi: 10.1177/11795484221105327. eCollection 2022. Clin Med Insights Circ Respir Pulm Med. 2022. PMID: 35693423 Free PMC article. Review.
Cited by
-
Antibody acquisition after second and third SARS-CoV-2 vaccinations in Japanese kidney transplant patients: a prospective study.Clin Exp Nephrol. 2023 Jun;27(6):574-582. doi: 10.1007/s10157-023-02334-0. Epub 2023 Mar 13. Clin Exp Nephrol. 2023. PMID: 36914824 Free PMC article.
-
Comparable cellular and humoral immunity upon homologous and heterologous COVID-19 vaccination regimens in kidney transplant recipients.Front Immunol. 2023 Mar 31;14:1172477. doi: 10.3389/fimmu.2023.1172477. eCollection 2023. Front Immunol. 2023. PMID: 37063863 Free PMC article.
-
Booster effect of the third dose of SARS-CoV-2 mRNA vaccine in Japanese kidney transplant recipients.Sci Rep. 2023 Jun 20;13(1):9976. doi: 10.1038/s41598-023-36998-1. Sci Rep. 2023. PMID: 37340001 Free PMC article.
-
Comparison of Humoral Response in Kidney Transplant Recipients and Donors and Healthy Volunteers Following Second Dose of SARS-CoV-2 mRNA Vaccine.Transplant Proc. 2023 Apr;55(3):514-520. doi: 10.1016/j.transproceed.2023.02.018. Epub 2023 Feb 27. Transplant Proc. 2023. PMID: 36948961 Free PMC article.
References
-
- Ben-Dov I. Z., Oster Y., Tzukert K., Alster T., Bader R., Israeli R., et al. . (2022). Impact of tozinameran (BNT162b2) mRNA vaccine on kidney transplant and chronic dialysis patients: 5 months (149.2 ±45.5 days) months follow-up. J. Nephrol. 35, 153–164. doi: 10.1007/s40620-021-01210-y, PMID: - DOI - PMC - PubMed
-
- Benotmane I., Bruel T., Planas D., Fafi-Kremer S., Schwartz O., Caillard S. (2022). A fourth dose of the mRNA-1273 SARS-CoV-2 vaccine improves serum neutralization against the delta variant in kidney transplant recipients. Kidney Int. 101, 1073–1076. doi: 10.1016/j.kint.2022.02.011, PMID: - DOI - PMC - PubMed
-
- Benotmane I., Gautier G., Perrin P., Olagne J., Cognard N., Fafi-Kremer S., et al. . (2021a). Antibodies response After a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients With minimal serologic response to 2 doses. JAMA 23:1065. doi: 10.1001/jama.2021.12339, PMID: - DOI - PMC - PubMed
-
- Benotmane I., Gautier-Vargas G., Cognard N., Olagne J., Heibel F., Braun-Parvez L., et al. . (2021b). Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 99, 1498–1500. doi: 10.1016/j.kint.2021.04.005, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous