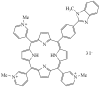
Interaction of 5-[4'-(N-Methyl-1,3-benzimidazol-2-yl)phenyl]-10,15,20-tri-(N-methyl-3'-pyridyl)porphyrin Triiodide with SARS-CoV-2 Spike Protein
- PMID: 35756101
- PMCID: PMC9207844
- DOI: 10.1134/S1070363222060123
Interaction of 5-[4'-(N-Methyl-1,3-benzimidazol-2-yl)phenyl]-10,15,20-tri-(N-methyl-3'-pyridyl)porphyrin Triiodide with SARS-CoV-2 Spike Protein
Abstract
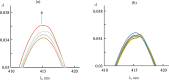
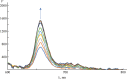
The results of experimental studies of the interaction of the S-protein with a monohetaryl-substituted porphyrin containing a benzimidazole residue are presented. It has been revealed that the S-protein forms high-affinity complexes with the specified porphyrin. The porphyrin binding by the SARS-CoV-2 S-protein has proceeded stepwise; at the first stage, the driving force of the complexation is electrostatic interaction between the surface negatively charged regions of the protein and cationic substituents of the porphyrin. At the second stage, the target complex of the S-protein with the porphyrin is formed. It has been established that the introduction of 5-[4'-(N-methyl-1,3-benzimidazol-2-yl)phenyl]-10,15,20-tri-(N-methyl-3'-pyridyl)porphyrin triiodide into a solution of the S-protein complex with the angiotensin-converting enzyme leads to the replacement of the latter with the porphyrin. Displacement of the angiotensin-converting enzyme from the complex with the S-protein under the action of 5-[4'-(N-methyl-1,3-benzimidazol-2-yl)phenyl]-10,15,20-tri-(N-methyl-3'-pyridyl)porphyrin triiodide is the experimental evidence for the porphyrin binding at the receptor-binding domain of the S-protein.
Keywords: SARS-CoV-2 virus; inactivation; inhibition; porphyrins; spectroscopy; spike protein.
© The Author(s) 2022.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
-
- Eggink, D., Andeweg, S.P., Vennema, H., van Maarseveen, N., Vermaas, K., Vlaemynck, B., Schepers, R., van Gageldonk-Lafeber, A.B., van den Hof, S., Reusken, C.B.E.M., and Knol, M.J., medRxiv., 2021. 10.1101/2021.12.20.21268121
-
- Rees-Spear C. Muir L., Griffith S.A., Heaney J., Aldon Y., Snitselaar J.L., Thomas P., Graham С., Seow J., Lee N., Rosa A., Roustan C., Houlihan C.F., Sanders R.W., Gupta R.K., Cherepanov P., Stauss H.J., Nastouli E., McCoy L.E. Cell Rep. 2021;34:108890. doi: 10.1016/j.celrep.2021.108890. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
