Neutrophil extracellular traps (NETs) reduce the diffusion of doxorubicin which may attenuate its ability to induce apoptosis of ovarian cancer cells
- PMID: 35756123
- PMCID: PMC9218137
- DOI: 10.1016/j.heliyon.2022.e09730
Neutrophil extracellular traps (NETs) reduce the diffusion of doxorubicin which may attenuate its ability to induce apoptosis of ovarian cancer cells
Abstract
Purpose: Although neutrophil extracellular traps (NETs) are present in various tumors, their roles in tumor biology have not been clarified yet. In this study, we examined how NETs affect the pharmacokinetics and effects of doxorubicin (DOX).
Methods: NETs were generated by neutrophils stimulated with phorbol 12-myristate 13-acetate (PMA) or lipopolysaccharide (LPS). DOX was added to NETs and their distribution was observed under fluorescein microscopy, and the diffusion of DOX through 3 μM pores from lower to upper chambers was evaluated with a fluorescence-based assay. Ovarian cancer cells, KOC-2S and SKOV3, were embedded in collagen gel droplets and cultured in 3D way and their apoptosis was examined with flow cytometry.
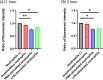
Results: DOX was mostly co-localized with NETs. The transfer of DOX to upper chambers increased over time, which was significantly decreased by the presence of neutrophils stimulated with PMA or LPS in the lower chamber. DOX outside of the gel increased the rates of annexin V (+) apoptotic cells, which were significantly reduced by the addition of LPS-stimulated neutrophils in media both in KOC-2S and SKOV3. The reduced diffusion and apoptosis were mostly restored by the destruction of the NETs structure with 1000 u/ml DNAse I.
Conclusion: NETs efficiently trap and inhibit the diffusion of DOX which may attenuate its ability to induce apoptosis of ovarian cancer cells. Degradation of NETs with DNAse I may augment the response of ovarian cancer to DOX.
Keywords: Chemosensitivity; Doxorubicin; Neutrophil extracellular traps; Pharmacokinetics.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Inhibitors of nitric oxide synthase can reduce extracellular traps from neutrophils in asthmatic children in vitro.Pediatr Pulmonol. 2020 Jan;55(1):68-75. doi: 10.1002/ppul.24520. Epub 2019 Oct 9. Pediatr Pulmonol. 2020. PMID: 31596059
-
Destruction of Neutrophil Extracellular Traps Promotes the Apoptosis and Inhibits the Invasion of Gastric Cancer Cells by Regulating the Expression of Bcl-2, Bax and NF-κB.Onco Targets Ther. 2020 Jun 9;13:5271-5281. doi: 10.2147/OTT.S227331. eCollection 2020. Onco Targets Ther. 2020. PMID: 32606746 Free PMC article.
-
[LPS stimulating neutrophils firmly adhered to ICAM-1 to form extracellular traps depends on integrin Mac-1 and cytoskeletal proteins].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021 Oct 25;38(5):903-910. doi: 10.7507/1001-5515.202105019. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021. PMID: 34713658 Free PMC article. Chinese.
-
Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis.Arthritis Rheum. 2012 Nov;64(11):3779-87. doi: 10.1002/art.34619. Arthritis Rheum. 2012. PMID: 22777766
-
Progression of Cystic Fibrosis Lung Disease from Childhood to Adulthood: Neutrophils, Neutrophil Extracellular Trap (NET) Formation, and NET Degradation.Genes (Basel). 2019 Feb 26;10(3):183. doi: 10.3390/genes10030183. Genes (Basel). 2019. PMID: 30813645 Free PMC article. Review.
Cited by
-
Increased formation of neutrophil extracellular traps in patients with anti-N-methyl-d-aspartate receptor encephalitis.Front Immunol. 2022 Dec 8;13:1046778. doi: 10.3389/fimmu.2022.1046778. eCollection 2022. Front Immunol. 2022. PMID: 36569875 Free PMC article.
-
Xuanfei Baidu Decoction regulates NETs formation via CXCL2/CXCR2 signaling pathway that is involved in acute lung injury.Biomed Pharmacother. 2023 May;161:114530. doi: 10.1016/j.biopha.2023.114530. Epub 2023 Mar 16. Biomed Pharmacother. 2023. PMID: 36933379 Free PMC article.
-
Ovarian cancer, neutrophil hitchhiking, and NETs: unraveling their role in pathogenesis and management.Med Oncol. 2025 Jun 30;42(8):302. doi: 10.1007/s12032-025-02860-9. Med Oncol. 2025. PMID: 40586945 Review.
-
Tumor‑associated neutrophils: Critical regulators in cancer progression and therapeutic resistance (Review).Int J Oncol. 2025 Apr;66(4):28. doi: 10.3892/ijo.2025.5734. Epub 2025 Feb 28. Int J Oncol. 2025. PMID: 40017131 Free PMC article. Review.
-
Coordination of Neutrophil and Apoptosis-Inducing Ligand in Inflammatory Diseases.J Inflamm Res. 2025 Mar 12;18:3607-3621. doi: 10.2147/JIR.S506807. eCollection 2025. J Inflamm Res. 2025. PMID: 40099000 Free PMC article. Review.
References
-
- Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013;13:159–175. - PubMed
-
- Gregory A.D., Houghton A.M. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. - PubMed
-
- Arelaki S., Arampatzioglou A., Kambas K., Papagoras C., Miltiades P., Angelidou I., Mitsios A., Kotsianidis I., Skendros P., Sivridis E., Maroulakou I., Giatromanolaki A., Ritis K. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PLoS One. 2016;11 - PMC - PubMed
LinkOut - more resources
Full Text Sources

