Organometallic catalysis in aqueous and biological environments: harnessing the power of metal carbenes
- PMID: 35756533
- PMCID: PMC9172117
- DOI: 10.1039/d2sc00721e
Organometallic catalysis in aqueous and biological environments: harnessing the power of metal carbenes
Abstract
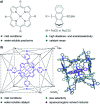
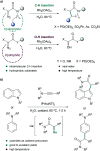
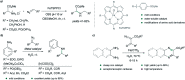
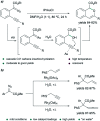
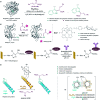
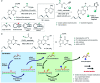
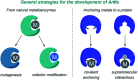
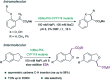
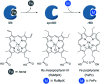
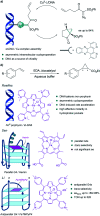
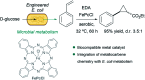
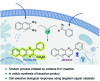
Translating the power of transition metal catalysis to the native habitats of enzymes can significantly expand the possibilities of interrogating or manipulating natural biological systems, including living cells and organisms. This is especially relevant for organometallic reactions that have shown great potential in the field of organic synthesis, like the metal-catalyzed transfer of carbenes. While, at first sight, performing metal carbene chemistry in aqueous solvents, and especially in biologically relevant mixtures, does not seem obvious, in recent years there has been a growing number of reports demonstrating the feasibility of the task. Either using small molecule metal catalysts or artificial metalloenzymes, a number of carbene transfer reactions that tolerate aqueous and biorelevant media are being developed. This review intends to summarize the most relevant contributions, and establish the state of the art in this emerging research field.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures







References
-
- Hartwig J. F., Organotransition metal chemistry: from bonding to catalysis, University Science Books: Mill Valley, CA, 2010
-
- Dixneuf P. H. and Cadierno V., Metal-catalyzed reactions in water, Wiley-VCH Verlag GmbH & Co. KGaA, 2013
Publication types
LinkOut - more resources
Full Text Sources

