Real World Overall Survival of Patients With Metastatic Renal Cell Carcinoma Treated With Only Available Sunitinib and Pazopanib in First-Line Setting
- PMID: 35756652
- PMCID: PMC9213683
- DOI: 10.3389/fonc.2022.892156
Real World Overall Survival of Patients With Metastatic Renal Cell Carcinoma Treated With Only Available Sunitinib and Pazopanib in First-Line Setting
Abstract
Background: The emerging new standard of care for metastatic clear cell renal carcinoma (mRCC) becomes a challenge when access to new drugs is limited. In Serbia, sunitinib and pazopanib are the only available first-line therapies. The second-line treatment for mRCC has never been and is still not available. We aimed to assess overall survival (OS) in patients with mRCC who received first-line sunitinib or pazopanib when access to second-line treatment was not available.
Methods: This retrospective observational study analyzed data from a nationally representative cohort of 759 patients who started on first-line sunitinib or pazopanib between 1 January 2012 and 30 June 2019, in 4 centers in Serbia. The data cut-off date was 31 December 2019. Key eligibility criteria were clear cell RCC histology, measurable metastatic disease, performance status 0 or 1, and the Memorial Sloan Kettering Cancer Center favorable or intermediate prognosis. The primary outcome was OS from the start of first-line treatment to death or data cut-off date.
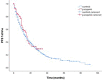
Results: The study population included 759 patients with mRCC who started with first-line sunitinib (n = 673; [88.7%]) or pazopanib (n = 86; [11.3%]). Overall, the mean age was 61.0 ± 9.7 years at treatment baseline, and 547 (72%) were men. mRCC was primarily diagnosed in 230 (30%) patients, and most of them underwent cytoreductive nephrectomy prior to systemic therapy (n = 181 [79%]). Additional treatment of metastases prior to and/or during treatment was used in 169 patients (22.3%). Grade 3 and 4 adverse events occurred in 168 (22.1%) and 47 patients (6.2%), respectively, and treatment was permanently stopped because of toxicity in 41 (6.9%). The OS was calculated from the start of first-line treatment, and the median follow-up was 14 months (range, 0-97). The median OS in the entire cohort was 17 months (95% CI, 14.6-19.4).
Conclusions: With only available sunitinib and pazopanib in first-line treatment, modest improvements are seen in the overall survival of patients with mRCC in real world clinical practice. In circumstances of limited availability of cancer medicines, our results can contribute to accelerating patient access to novel cancer therapies that have been shown to prolong survival in mRCC.
Keywords: limited access to cancer therapy; metastatic clear cell renal carcinoma (mRCC); overall survival (OS); pazopanib; real world data; sunitinib.
Copyright © 2022 Nikic, Babovic, Dzamic, Salma, Stojanovic, Matkovic, Pejcic, Juskic and Soldatovic.
Conflict of interest statement
PN, NB, ZD, SS, VS, and SM received honoraria for lectures and travel grants from Pfizer and Novartis outside the submitted work. No other disclosures were reported. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abu-Ghanem Y, Powles T, Capitanio U, Beisland C, Järvinen P, Stewart GD, et al. The Impact of Histological Subtype on the Incidence, Timing, and Patterns of Recurrence in Patients With Renal Cell Carcinoma After Surgery-Results From RECUR Consortium. Eur Urol Oncol (2021) 4(3):473–82. doi: 10.1016/j.euo.2020.09.005 - DOI - PubMed
-
- National Comprehensive Cancer Network . Kidney Cancer (Version 4.2022). Available at: https://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf (Accessed January 19, 2022).
-
- Chakiryan NH, Jiang DD, Gillis KA, Green E, Hajiran A, Hugar L, et al. Real-World Survival Outcomes Associated With First-Line Immunotherapy, Targeted Therapy, and Combination Therapy for Metastatic Clear Cell Renal Cell Carcinoma. JAMA Netw Open (2021) 4(5):e2111329. doi: 10.1001/jamanetworkopen.2021.11329 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources