Solution- and gas-phase behavior of decavanadate: implications for mass spectrometric analysis of redox-active polyoxidometalates
- PMID: 35756945
- PMCID: PMC9216222
- DOI: 10.1039/d1qi01618k
Solution- and gas-phase behavior of decavanadate: implications for mass spectrometric analysis of redox-active polyoxidometalates
Abstract
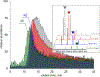
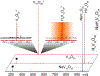
Decavanadate (V10O28 6- or V10) is a paradigmatic member of the polyoxidometalate (POM) family, which has been attracting much attention within both materials/inorganic and biomedical communities due to its unique structural and electrochemical properties. In this work we explored the utility of high-resolution electrospray ionization (ESI) mass spectrometry (MS) and ion exclusion chromatography LC/MS for structural analysis of V10 species in aqueous solutions. While ESI generates abundant molecular ions representing the intact V10 species, their isotopic distributions show significant deviations from the theoretical ones. A combination of high-resolution MS measurements and hydrogen/deuterium exchange allows these deviations to be investigated and interpreted as a result of partial reduction of V10. While the redox processes are known to occur in the ESI interface and influence the oxidation state of redox-active analytes, the LC/MS measurements using ion exclusion chromatography provide unequivocal evidence that the mixed-valence V10 species exist in solution, as extracted ion chromatograms representing V10 molecular ions at different oxidation states exhibit distinct elution profiles. The spontaneous reduction of V10 in solution is seen even in the presence of hydrogen peroxide and has not been previously observed. The susceptibility to reduction of V10 is likely to be shared by other redox active POMs. In addition to the molecular V10 ions, a high-abundance ionic signal for a V10O26 2- anion was displayed in the negative-ion ESI mass spectra. None of the V10O26 cations were detected in ESI MS, and only a low-abundance signal was observed for V10O26 anions with a single negative charge, indicating that the presence of abundant V10O26 2- anions in ESI MS reflects gas-phase instability of V10O28 anions carrying two charges. The gas-phase origin of the V10O26 2- anion was confirmed in tandem MS measurements, where mild collisional activation was applied to V10 molecular ions with an even number of hydrogen atoms (H4V10O28 2-), resulting in a facile loss of H2O molecules and giving rise to V10O26 2- as the lowest-mass fragment ion. Water loss was also observed for V10O28 anions carrying an odd number of hydrogen atoms (e.g., H5V10O28 -), followed by a less efficient and incomplete removal of an OH• radical, giving rise to both HV10O26 - and V10O25 - fragment ions. Importantly, at least one hydrogen atom was required for ion fragmentation in the gas phase, as no further dissociation was observed for any hydrogen-free V10 ionic species. The presented workflow allows a distinction to be readily made between the spectral features revealing the presence of non-canonical POM species in the bulk solution from those that arise due to physical and chemical processes occurring in the ESI interface and/or the gas phase.
Figures

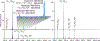
References
-
- Müller A; Peters F; Pope MT; Gatteschi D Polyoxometalates: Very Large ClustersNanoscale Magnets. Chem. Rev 1998, 98, 239–272. - PubMed
-
- Gouzerh P; Che M From Scheele and Berzelius to Muller - Polyoxometalates (POMs) revisited and the “missing link” between the bottom up and top down approaches. Actual Chim. 2006, 9–22.
-
- Hill CL Progress and challenges in polyoxometalate-based catalysis and catalytic materials chemistry. J. Mol. Catal. A-Chem 2007, 262, 2–6.
-
- Vila-Nadal L; Mitchell SG; Markov S; Busche C; Georgiev V; Asenov A; Cronin L Towards Polyoxometalate-Cluster-Based Nano-Electronics. Chem.-Eur. J 2013, 19, 16502–16511. - PubMed
-
- Wang SM; Hwang J; Kim E Polyoxometalates as promising materials for electrochromic devices. J. Mater. Chem. C 2019, 7, 7828–7850.
Grants and funding
LinkOut - more resources
Full Text Sources
