Probing the growth and mechanical properties of Bacillus subtilis biofilms through genetic mutation strategies
- PMID: 35756965
- PMCID: PMC9194759
- DOI: 10.1016/j.synbio.2022.05.005
Probing the growth and mechanical properties of Bacillus subtilis biofilms through genetic mutation strategies
Abstract
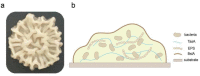
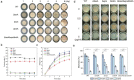
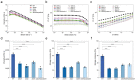
Bacterial communities form biofilms on various surfaces by synthesizing a cohesive and protective extracellular matrix, and these biofilms protect microorganisms against harsh environmental conditions. Bacillus subtilis is a widely used experimental species, and its biofilms are used as representative models of beneficial biofilms. Specifically, B. subtilis biofilms are known to be rich in extracellular polymeric substances (EPS) and other biopolymers such as DNA and proteins like the amyloid protein TasA and the hydrophobic protein BslA. These materials, which form an interconnected, cohesive, three-dimensional polymer network, provide the mechanical stability of biofilms and mediate their adherence to surfaces among other functional contributions. Here, we explored how genetically-encoded components specifically contribute to regulate the growth status, mechanical properties, and antibiotic resistance of B. subtilis biofilms, thereby establishing a solid empirical basis for understanding how various genetic engineering efforts are likely to affect the structure and function of biofilms. We noted discrete contributions to biofilm morphology, mechanical properties, and survival from major biofilm components such as EPS, TasA and BslA. For example, EPS plays an important role in maintaining the stability of the mechanical properties and the antibiotic resistance of biofilms, whereas BslA has a significant impact on the resolution that can be obtained for printing applications. This work provides a deeper understanding of the internal interactions of biofilm components through systematic genetic manipulations. It thus not only broadens the application prospects of beneficial biofilms, but also serves as the basis of future strategies for targeting and effectively removing harmful biofilms.
Keywords: Bacillus subtilis; Biofilms; Environmental tolerance; Living materials; Synthetic biology.
© 2022 The Authors.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Flemming H.C., et al. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563–575. - PubMed
-
- Flemming H.C., Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–633. - PubMed
-
- Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. - PubMed
LinkOut - more resources
Full Text Sources

