Retinal Neurodegeneration in Different Risk Phenotypes of Diabetic Retinal Disease
- PMID: 35757010
- PMCID: PMC9231566
- DOI: 10.3389/fnins.2021.800004
Retinal Neurodegeneration in Different Risk Phenotypes of Diabetic Retinal Disease
Abstract
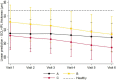
Diabetic retinopathy (DR) has been considered a microvascular disease, but it has become evident that neurodegeneration also plays a key role in this complex pathology. Indeed, this complexity is reflected in its progression which occurs at different rates in different type 2 diabetic (T2D) individuals. Based on this concept, our group has identified three DR progression phenotypes that might reflect the interindividual differences: phenotype A, characterized by low microaneurysm turnover (MAT <6), phenotype B, low MAT (<6) and increased central retinal thickness (CRT); and phenotype C, with high MAT (≥6). In this study, we evaluated the progression of DR neurodegeneration, considering ganglion cell+inner plexiform layers (GCL+IPL) thinning, in 170 T2D individuals followed for a period of 5 years, to explore associations with disease progression or risk phenotypes. Ophthalmological examinations were performed at baseline, first 6 months, and annually. GCL+IPL average thickness was evaluated by optical coherence tomography (OCT). Microaneurysm turnover (MAT) was evaluated using the RetMarkerDR. ETDRS level and severity progression were assessed in seven-field color fundus photography. In the overall population there was a significant loss in GCL+IPL (-0.147 μm/year), independently of glycated hemoglobin, age, sex, and duration of diabetes. Interestingly, this progressive thinning in GCL + IPL reached higher values in phenotypes B and C (-0.249 and -0.238 μm/year, respectively), whereas phenotype A remained relatively stable. The presence of neurodegeneration in all phenotypes suggests that it is the retinal vascular response to the early neurodegenerative changes that determines the course of the retinopathy in each individual. Therefore, classification of different DR phenotypes appears to offer relevant clarification of DR disease progression and an opportunity for improved management of each T2D individual with DR, thus playing a valuable role for the implementation of personalized medicine in DR.
Keywords: diabetes; neurodegeneration; personalized medicine; progression; retinopathy; risk phenotypes.
Copyright © 2021 Madeira, Marques, Ferreira, Tavares, Santos, Santos, Figueira, Lobo and Cunha-Vaz.
Conflict of interest statement
JF reports being a member of Advisory Boards for Alimera, Allergan, Bayer, Bhoeringer, and No-vartis. JC-V reports being a consultant for Alimera Sciences, Allergan, Bayer, Gene Signal, Novartis, Pfizer, Precision Ocular Ltd., Roche, Sanofi-Aventis, Vifor Pharma, and Carl Zeiss Meditec. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Cunha-Vaz J., Bernardes R., Santos T., Oliveira C. M., Lobo C., Pires I., et al. (2012). Computer-aided detection of diabetic retinopathy progression. Digital Teleret. Screen. Teleophthalmol. Pract. 226 161–181. 10.1007/978-3-642-25810-7_6 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

