Photo-degradable, tough and highly stretchable hydrogels
- PMID: 35757031
- PMCID: PMC9218832
- DOI: 10.1016/j.mtbio.2022.100325
Photo-degradable, tough and highly stretchable hydrogels
Abstract
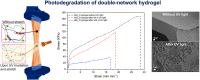
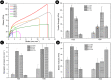
We present for the first time highly stretchable and tough hydrogels with controlled light-triggered photodegradation. A double-network of alginate/polyacrylamide (PAAm) is formed by using covalently and ionically crosslinked subnetworks. The ionic Ca2+ alginate interpenetrates a PAAm network covalently crosslinked by a bifunctional acrylic crosslinker containing the photodegradable o-nitrobenzyl (ONB) core instead of the commonly used methylene bisacrylamide (MBAA). Remarkably, due to the developed protocol, the change of the crosslinker did not affect the hydrogel's mechanical properties. The incorporation of photosensitive components in hydrogels allows external temporal control of their properties and tuneable degradation. Cell viability and cell proliferation assays revealed that hydrogels and their photodegradation products are not cytotoxic to the NIH3T3 cell line. In one example of application, we used these hydrogels for bio-potential acquisition in wearable electrocardiography. Surprisingly, these hydrogels showed a lower skin-electrode impedance, compared to the common medical grade Ag/AgCl electrodes. This work lays the foundation for the next generation of tough and highly stretchable hydrogels that are environmentally friendly and can find applications in a variety of fields such as health, electronics, and energy, as they combine excellent mechanical properties with controlled degradation.
Keywords: Double-network; Hydrogel; Stretchable light-degradable; Tough; o-nitrobenzyl.
© 2022 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

