10-Year Impact of Transcatheter Aortic Valve Replacement Leaflet Design (Intra- Versus Supra-Annular) in Mortality and Hemodynamic Performance
- PMID: 35757343
- PMCID: PMC9215259
- DOI: 10.3389/fcvm.2022.924958
10-Year Impact of Transcatheter Aortic Valve Replacement Leaflet Design (Intra- Versus Supra-Annular) in Mortality and Hemodynamic Performance
Abstract
Background: The impact of transcatheter aortic valve replacement (TAVR) leaflet design on long-term device performance is still unknown. This study sought to compare the clinical and hemodynamic outcomes of intra- (IA) versus supra-annular (SA) TAVR designs up-to 10-years following implantation.
Methods: Consecutive patients with at least 5-years follow-up following TAVR for severe symptomatic aortic stenosis from June 2007 to December 2016 were included. Bioprosthetic valve failure (BVF) and hemodynamic valve deterioration (HVD) were defined according to VARC-3 updated definitions and estimated using cumulative incidence function to account for the competing risk of death.
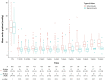
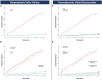
Results: A total of 604 patients (82 years; 53% female) were analyzed and divided into IA (482) and SA (122) groups. Overall survival rates at 10-years were similar (IA 15%, 95%CI: 10-22; SA 11%, 95%CI: 6-20; p = 0.21). Compared to the SA TAVR, mean transaortic gradients were significantly higher and increased over time in the IA group. IA TAVRs showed higher 10-year cumulative incidences of BVF (IA 8% vs. SA 1%, p = 0.02) and severe HVD (IA 5% vs. SA 1%, p = 0.05). The occurrence of BVF and HVD in the IA group occurred primarily in the smallest TAVR devices (20-23-mm). After excluding these sizes, the cumulative incidences of BVF (IA 5% vs. SA 1%, p = 0.40) and severe HVD (IA 2% vs. SA 1%, p = 0.11) were similar.
Conclusion: In this study, TAVR leaflet design had no impact on survival at 10-years. IA devices showed higher transaortic gradients and cumulative incidences of HVD and BVF predominantly occurring in the smallest valve sizes.
Keywords: bioprosthetic valve failure; hemodynamic valve deterioration; intra-annular; supra-annular; transcatheter aortic valve replacement.
Copyright © 2022 Scotti, Fovino, Coisne, Fabris, Cardaioli, Massussi, Rodinò, Barolo, Boiago, Continisio, Montonati, Sciarretta, Zuccarelli, Bernardini, Masiero, Napodano, Fraccaro, Marchese, Esposito, Granada, Latib, Iliceto and Tarantini.
Conflict of interest statement
GT reports honoraria for lectures/consulting from Medtronic, Edwards Lifesciences, Boston Scientific, and Abbott. AL is an advisor and reports honoraria for consulting from Medtronic, Edwards Lifesciences, Boston Scientific, and Abbott. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

