Severe Hyperprolactinemia Promotes Brown Adipose Tissue Whitening and Aggravates High Fat Diet Induced Metabolic Imbalance
- PMID: 35757410
- PMCID: PMC9226672
- DOI: 10.3389/fendo.2022.883092
Severe Hyperprolactinemia Promotes Brown Adipose Tissue Whitening and Aggravates High Fat Diet Induced Metabolic Imbalance
Abstract
Background: The association of high serum prolactin and increased body weight is positive but controversial, therefore we hypothesized that additional factors such as diets and the impact of prolactin on brown adipose tissue may condition its metabolic effects.
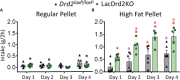
Methods: We used LacDrd2KO females with lifelong severe hyperprolactinemia due dopamine-D2 receptor deletion from lactotropes, and slow onset of metabolic disturbances, and compared them to their respective controls (Drd2 loxP/loxP ). Food intake, and binge eating was evaluated. We then challenged mice with a High Fat (HFD) or a Control Diet (CD) for 8 weeks, beginning at 3 months of age, when no differences in body weight are found between genotypes. At the end of the protocol brown and white adipose tissues were weighed, and thermogenic and lipogenic markers studied, using real time PCR (Ucp1, Cidea, Pgc1a, Lpl, adiponectin, Prlr) or immunohistochemistry (UCP1). Histochemical analysis of brown adipose tissue, and glucose tolerance tests were performed.
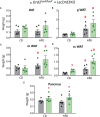
Results: Hyperprolactinemic mice had increased food intake and binge eating behavior. Metabolic effects induced by a HFD were exacerbated in lacDrd2KO mice. Hyperprolactinemia aggravated HFD-induced body weight gain and glucose intolerance. In brown adipose tissue pronounced cellular whitening as well as decreased expression of the thermogenic markers Ucp1 and Pgc1a were observed in response to high prolactin levels, regardless of the diet, and furthermore, hyperprolactinemia potentiated the decrease in Cidea mRNA expression induced by HFD. In subcutaneous white adipose tissue hyperprolactinemia synergistically increased tissue weight, while decreasing Prlr, Adiponectin and Lpl mRNA levels regardless of the diet.
Conclusions: Pathological hyperprolactinemia has a strong impact in brown adipose tissue, lowering thermogenic markers and evoking tissue whitening. Furthermore, it modifies lipogenic markers in subcutaneous white adipose, and aggravates HFD-induced glucose intolerance and Cidea decrease. Therefore, severe high prolactin levels may target BAT function, and furthermore represent an adjuvant player in the development of obesity induced by high fat diets.
Keywords: CIDEA; PGC1 alpha; UCP1; brown adipose tissue; obesity; prolactin; thermogenic markers; whitening.
Copyright © 2022 Lopez-Vicchi, De Winne, Ornstein, Sorianello, Toneatto and Becu-Villalobos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

