Gut Bacteria Regulate the Pathogenesis of Huntington's Disease in Drosophila Model
- PMID: 35757549
- PMCID: PMC9215115
- DOI: 10.3389/fnins.2022.902205
Gut Bacteria Regulate the Pathogenesis of Huntington's Disease in Drosophila Model
Erratum in
-
Corrigendum: Gut bacteria regulate the pathogenesis of Huntington's disease in Drosophila model.Front Neurosci. 2022 Oct 13;16:991513. doi: 10.3389/fnins.2022.991513. eCollection 2022. Front Neurosci. 2022. PMID: 36312028 Free PMC article.
Abstract
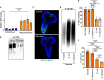
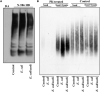
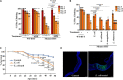
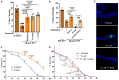
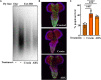
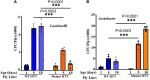
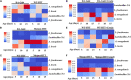
Changes in the composition of gut microbiota are implicated in the pathogenesis of several neurodegenerative disorders. Here, we investigated whether gut bacteria affect the progression of Huntington's disease (HD) in transgenic Drosophila melanogaster (fruit fly) models expressing full-length or N-terminal fragments of human mutant huntingtin (HTT) protein. We find that elimination of commensal gut bacteria by antibiotics reduces the aggregation of amyloidogenic N-terminal fragments of HTT and delays the development of motor defects. Conversely, colonization of HD flies with Escherichia coli (E. coli), a known pathobiont of human gut with links to neurodegeneration and other morbidities, accelerates HTT aggregation, aggravates immobility, and shortens lifespan. Similar to antibiotics, treatment of HD flies with small compounds such as luteolin, a flavone, or crocin a beta-carotenoid, ameliorates disease phenotypes, and promotes survival. Crocin prevents colonization of E. coli in the gut and alters the levels of commensal bacteria, which may be linked to its protective effects. The opposing effects of E. coli and crocin on HTT aggregation, motor defects, and survival in transgenic Drosophila models support the involvement of gut-brain networks in the pathogenesis of HD.
Keywords: Huntington’s disease; crocin (PubChem CID: 5281233); gut-brain; microbiota; neurodegeneration.
Copyright © 2022 Chongtham, Yoo, Chin, Akingbesote, Huda, Marsh and Khoshnan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

