Clinical Significance of Serum Albumin and Implications of FcRn Inhibitor Treatment in IgG-Mediated Autoimmune Disorders
- PMID: 35757719
- PMCID: PMC9231186
- DOI: 10.3389/fimmu.2022.892534
Clinical Significance of Serum Albumin and Implications of FcRn Inhibitor Treatment in IgG-Mediated Autoimmune Disorders
Abstract
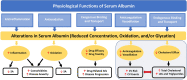
Serum albumin (SA), the most abundant soluble protein in the body, maintains plasma oncotic pressure and regulates the distribution of vascular fluid and has a range of other important functions. The goals of this review are to expand clinical knowledge regarding the functions of SA, elucidate effects of dysregulated SA concentration, and discuss the clinical relevance of hypoalbuminemia resulting from various diseases. We discuss potential repercussions of SA dysregulation on cholesterol levels, liver function, and other processes that rely on its homeostasis, as decreased SA concentration has been shown to be associated with increased risk for cardiovascular disease, hyperlipidemia, and mortality. We describe the anti-inflammatory and antioxidant properties of SA, as well as its ability to bind and transport a plethora of endogenous and exogenous molecules. SA is the primary serum protein involved in binding and transport of drugs and as such has the potential to affect, or be affected by, certain medications. Of current relevance are antibody-based inhibitors of the neonatal Fc receptor (FcRn), several of which are under clinical development to treat immunoglobulin G (IgG)-mediated autoimmune disorders; some have been shown to decrease SA concentration. FcRn acts as a homeostatic regulator of SA by rescuing it, as well as IgG, from intracellular degradation via a common cellular recycling mechanism. Greater clinical understanding of the multifunctional nature of SA and the potential clinical impact of decreased SA are needed; in particular, the potential for certain treatments to reduce SA concentration, which may affect efficacy and toxicity of medications and disease progression.
Keywords: FcRn; IgG; albumin; autoimmune; hypoalbuminemia; monoclonal antibody; serum protein.
Copyright © 2022 Ward, Gelinas, Dreesen, Van Santbergen, Andersen, Silvestri, Kiss, Sleep, Rader, Kastelein, Louagie, Vidarsson and Spriet.
Conflict of interest statement
DG, EL, and JVS are employees of argenx, the manufacturer of efgartigimod (FDA approved in gMG and under investigation in primary immune thrombocytopenia, pemphigus vulgaris and foliaceus, chronic inflammatory demyelinating polyneuropathy, bullous pemphigoid, and idiopathic inflammatory myopathy [myositis]). JTA has received funding from Syntimmune and argenx as part of fee-for-service agreements. ED is a postdoctoral research fellow of the Research Foundation – Flanders (grant number 12X9420N). JEK has participated on the Medical Advisory Committee of Sanofi Genzyme and Plasma Advisory Committee of Haemonetics. DR is the founder of Staten Biotechnology. NS is a member of the scientific advisory board and a speaker for argenx. IS, is supported by the Clinical Research Fund of the University Hospitals Leuven. ESW may receive royalties from patents owned by the UK Medical Research Council, UT Southwestern Medical Center, and Texas A&M University, and has financial interests in argenx, Astero BioPharma LLC, Astero Erado Inc., and Astero Technologies LLC. GV is a paid consultant for argenx. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The development (medical writing and editorial support) of this manuscript received funding from argenx. Authors who are not affiliated with argenx were not paid for their contributions to this work. The funder was involved in conception of design, interpretation of data, writing of this article, and decision to submit it for publication.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

