Global Expansion of Jeffrey's Insights: Jeffrey Modell Foundation's Genetic Sequencing Program for Primary Immunodeficiency
- PMID: 35757720
- PMCID: PMC9226364
- DOI: 10.3389/fimmu.2022.906540
Global Expansion of Jeffrey's Insights: Jeffrey Modell Foundation's Genetic Sequencing Program for Primary Immunodeficiency
Abstract
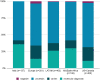
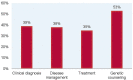
Genetic disorders that impair the immune system, known as Primary Immunodeficiencies (PI), include over 450 single-gene inborn errors of immunity. Timely and appropriate diagnosis and treatment is vital to quality of life (QOL) and sometimes survival, as patients are susceptible to frequent, persistent, severe, and sometimes life-threatening infections or autoimmunity. Suspected PI patients that do not have a genetic diagnosis often endure a prolonged, onerous, inefficient, and expensive experience, known as a diagnostic odyssey. The resulting diagnostic delay prohibits proper disease management and treatment, causing unnecessary distress and diminished QOL. Next-generation sequencing (NGS) offers relief from the distress of the diagnostic odyssey, but because of cost and barriers to access, it is regularly unobtainable. The Jeffrey Modell Foundation (JMF) introduced "Jeffrey's Insights", a no-charge genetic sequencing pilot program, in January 2019 for patients within the Jeffrey Modell Centers Network (JMCN) with an underlying PI, but no genetic diagnosis. Building on the success of the pilot program, JMF expanded it globally to more than 400 Centers in the JMCN in early 2020. The most current version of Invitae's PI Panel available was used for this program. All participating clinicians were invited to complete a brief questionnaire assessing prior impediments to access and post-sequencing alterations in disease management and treatment. A total of 1,398 patients were tested, with 20.3% receiving a molecular diagnosis and many more receiving helpful diagnostic leads. Results obtained from genetic sequencing led to an alteration of clinical diagnosis, disease management, treatment, and genetic counseling in 39%, 38%, 35%, and 53% of patients, respectively. The global expansion of this program further underscores the crucial need for NGS for PI, along with its efficiency and potential cost savings. The results of this program to date further define rationale for the availability of comprehensive diagnostic NGS for patients with PI when requisitioned by an expert immunologist.
Keywords: Inborn Errors of Immunity (IEI); Jeffrey Modell Centers Network (JMCN); Jeffrey Modell Foundation (JMF); Next Generation Sequencing (NGS); Primary Immunodeficiency (PI); genetic sequencing; sequencing.
Copyright © 2022 Quinn, Modell, Johnson, Poll, Aradhya, Orange and Modell.
Conflict of interest statement
JO: Consultant to Grifols, CSL, Takeda, Teva, Sobi, Jansen, Editas; ADMA, Gigagen, and Edity Scientific Advisory Boards; author and editor in immunology for Up To Date receiving royalties; patent related to genetic testing held by Children’s Hospital of Philadelphia. SP, SA, and BJ are current salaried employees of Invitae, including stock benefits. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- National Institute of Allergy and Infectious Diseases . Primary Immune Deficiency Diseases. Available at: https://www.niaid.nih.gov/diseases-conditions/primary-immune-deficiency-... (Accessed May 3, 2021).
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

