mTORC1 Inhibition Protects Human Regulatory T Cells From Granzyme-B-Induced Apoptosis
- PMID: 35757726
- PMCID: PMC9229986
- DOI: 10.3389/fimmu.2022.899975
mTORC1 Inhibition Protects Human Regulatory T Cells From Granzyme-B-Induced Apoptosis
Abstract
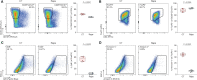
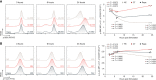
Regulatory T cells (Tregs) have shown great promise as a means of cellular therapy in a multitude of allo- and auto-immune diseases-due in part to their immunosuppressive potency. Nevertheless, the clinical efficacy of human Tregs in patients has been limited by their poor in vivo homeostasis. To avert apoptosis, Tregs require stable antigenic (CD3ζ/T-cell-receptor-mediated), co-stimulatory (CD28-driven), and cytokine (IL-2-dependent) signaling. Notably, this sequence of signals supports an activated Treg phenotype that includes a high expression of granzymes, particularly granzyme B (GrB). Previously, we have shown that aside from the functional effects of GrB in lysing target cells to modulate allo-immunity, GrB can leak out of the intracellular lysosomal granules of host Tregs, initiating pro-apoptotic pathways. Here, we assessed the role of inhibiting mechanistic target of rapamycin complex 1 (mTORC1), a recently favored drug target in the transplant field, in regulating human Treg apoptosis via GrB. Using ex vivo models of human Treg culture and a humanized mouse model of human skin allotransplantation, we found that by inhibiting mTORC1 using rapamycin, intracytoplasmic expression and functionality of GrB diminished in host Tregs; lowering human Treg apoptosis by in part decreasing the phosphorylation of S6K and c-Jun. These findings support the already clinically validated effects of mTORC1 inhibition in patients, most notably their stabilization of Treg bioactivity and in vivo homeostasis.
Keywords: granzyme B; grapoptosis; human tregs; mTORC1; rapamycin; treg homeostasis.
Copyright © 2022 Eskandari, Allos, Al Dulaijan, Melhem, Sulkaj, Alhaddad, Saad, Deban, Chu, Choi, Kollar, Pomahac, Riella, Berger, Sanders, Lieberman, Li and Azzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Gershon RK, Cohen P, Hencin R, Liebhaber SA. Suppressor T Cells. J Immunol (1972) 108:586–90. - PubMed
-
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor Alpha-Chains (CD25). Breakdown of a Single Mechanism of Self-Tolerance Causes Various Autoimmune Diseases. J Immunol (1995) 155:1151–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

