Case Report: Azathioprine: An Old and Wronged Immunosuppressant
- PMID: 35757730
- PMCID: PMC9226564
- DOI: 10.3389/fimmu.2022.903012
Case Report: Azathioprine: An Old and Wronged Immunosuppressant
Abstract
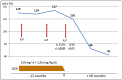
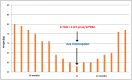
Mycophenolate rapidly substituted azathioprine (AZA) in transplant immunosuppression regimens since the 1990s, when early clinical trials indicated better outcomes, although opposite results were also observed. However, none of these trials used the well-established optimization methods for AZA dosing, namely, thiopurine methyltransferase pharmacogenetics combined with monitoring of the thiopurine metabolites 6-thioguanine nucleotides (6-TGN) and 6-methylmercaptopurine (6-MMP). Resistance to optimize AZA therapy remains today in transplant therapy, despite the fact that thiopurine metabolite testing is being used by other medical disciplines with evident improvement in clinical results. In a previous analysis, we found that active 6-TGN metabolites were not detectable in about 30% of kidney transplant patients under continuous use of apparently adequate azathioprine dosage, which demonstrates the need to monitor these metabolites for therapeutic optimization. Two of four case studies presented here exemplifies this fact. On the other hand, some patients have toxic 6-TGN levels with a theoretically appropriate dose, as seen in the other two case studies in this presentation, constituting one more important reason to monitor the AZA dose administered by its metabolites. This analysis is not intended to prove the superiority of one immunosuppressant over another, but to draw attention to a fact: there are thousands of patients around the world receiving an inadequate dose of azathioprine and, therefore, with inappropriate immunosuppression. This report is also intended to draw attention, to clinicians using thiopurines, that allopurinol co-therapy with AZA is a useful therapeutic pathway for those patients who do not adequately form active thioguanine metabolites.
Keywords: 6-TGN; allopurinol; azathioprine; metabolites; mycophenolate; renal transplant.
Copyright © 2022 Chocair, Neves, Mohrbacher, Neto, Sato, Oliveira, Barbosa, Bales, Silva, Cuvello-Neto and Duley.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Determination of 6-thioguanine and 6-methylmercaptopurine metabolites in renal transplantation recipients and patients with glomerulonephritis treated with azathioprine.Ther Drug Monit. 1999 Apr;21(2):231-7. doi: 10.1097/00007691-199904000-00015. Ther Drug Monit. 1999. PMID: 10217345
-
Febuxostat as a novel option to optimize thiopurines' metabolism in patients with inadequate metabolite levels.Ann Pharmacother. 2014 May;48(5):648-51. doi: 10.1177/1060028014521389. Epub 2014 Feb 12. Ann Pharmacother. 2014. PMID: 24523395
-
Should 6-thioguanine nucleotides be monitored in heart transplant recipients given azathioprine?Ther Drug Monit. 1996 Jun;18(3):228-33. doi: 10.1097/00007691-199606000-00002. Ther Drug Monit. 1996. PMID: 8738760 Clinical Trial.
-
Review article: the benefits of pharmacogenetics for improving thiopurine therapy in inflammatory bowel disease.Aliment Pharmacol Ther. 2012 Jan;35(1):15-36. doi: 10.1111/j.1365-2036.2011.04905.x. Epub 2011 Nov 2. Aliment Pharmacol Ther. 2012. PMID: 22050052 Review.
-
[Monitoring of thiopurine methyltransferase and thiopurine metabolites to optimize azathioprine therapy in inflammatory bowel disease].Gastroenterol Hepatol. 2006 Nov;29(9):568-83. doi: 10.1157/13094355. Gastroenterol Hepatol. 2006. PMID: 17129552 Review. Spanish.
Cited by
-
Current trends in acute pancreatitis: Diagnostic and therapeutic challenges.World J Gastroenterol. 2023 May 14;29(18):2747-2763. doi: 10.3748/wjg.v29.i18.2747. World J Gastroenterol. 2023. PMID: 37274068 Free PMC article. Review.
-
Antiviral Potential of Azathioprine and Its Derivative 6- Mercaptopurine: A Narrative Literature Review.Pharmaceuticals (Basel). 2024 Jan 30;17(2):174. doi: 10.3390/ph17020174. Pharmaceuticals (Basel). 2024. PMID: 38399389 Free PMC article. Review.
References
-
- Chocair PR, Duley JA, Sabbaga E, Arap S, Simmonds HA, Cameron JS. Fast and Slow Methylators: Do Racial Differences Influence Risk of Allograft Rejection? Quart J Med (1993) 86(6):359–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous