Differential Biodistribution of Adenoviral-Vectored Vaccine Following Intranasal and Endotracheal Deliveries Leads to Different Immune Outcomes
- PMID: 35757753
- PMCID: PMC9231681
- DOI: 10.3389/fimmu.2022.860399
Differential Biodistribution of Adenoviral-Vectored Vaccine Following Intranasal and Endotracheal Deliveries Leads to Different Immune Outcomes
Erratum in
-
Corrigendum: Differential biodistribution of adenoviral-vectored vaccine following intranasal and endotracheal deliveries leads to different immune outcomes.Front Immunol. 2023 Feb 7;14:1151809. doi: 10.3389/fimmu.2023.1151809. eCollection 2023. Front Immunol. 2023. PMID: 36825013 Free PMC article.
Abstract
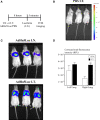
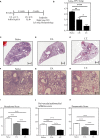
Infectious diseases of the respiratory tract are one of the top causes of global morbidity and mortality with lower respiratory tract infections being the fourth leading cause of death. The respiratory mucosal (RM) route of vaccine delivery represents a promising strategy against respiratory infections. Although both intranasal and inhaled aerosol methods have been established for human application, there is a considerable knowledge gap in the relationship of vaccine biodistribution to immune efficacy in the lung. Here, by using a murine model and an adenovirus-vectored model vaccine, we have compared the intranasal and endotracheal delivery methods in their biodistribution, immunogenicity and protective efficacy. We find that compared to intranasal delivery, the deepened and widened biodistribution in the lung following endotracheal delivery is associated with much improved vaccine-mediated immunogenicity and protection against the target pathogen. Our findings thus support further development of inhaled aerosol delivery of vaccines over intranasal delivery for human application.
Keywords: Adenovirus-vectored vaccine; T cells; Tuberculosis; biodistribution; endotracheal; intranasal; mucosal immunity; respiratory mucosal immunization.
Copyright © 2022 Jeyananthan, Afkhami, D’Agostino, Zganiacz, Feng, Miller, Jeyanathan, Thompson and Xing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- World Health Organization . WHO - The Top 10 Causes of Death. (2018). pp. 1–7. Available at: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of... [Accessed May 25, 2022].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

