An ADAM17-Neutralizing Antibody Reduces Inflammation and Mortality While Increasing Viral Burden in a COVID-19 Mouse Model
- PMID: 35757773
- PMCID: PMC9226444
- DOI: 10.3389/fimmu.2022.918881
An ADAM17-Neutralizing Antibody Reduces Inflammation and Mortality While Increasing Viral Burden in a COVID-19 Mouse Model
Abstract
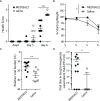
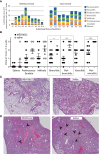
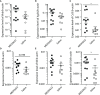
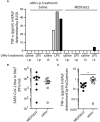
Angiotensin Converting Enzyme 2 (ACE2) is the primary cell entry receptor for SARS-CoV and SARS-CoV-2 viruses. A disintegrin and metalloproteinase 17 (ADAM17) is a protease that cleaves ectodomains of transmembrane proteins, including that of ACE2 and the proinflammatory cytokine TNF-α, from cell surfaces upon cellular activation. We hypothesized that blockade of ADAM17 activity would alter COVID-19 pathogenesis. To assess this pathway, we blocked the function of ADAM17 using the monoclonal antibody MEDI3622 in the K18-hACE2 transgenic mouse model of COVID-19. Antibody-treated mice were healthier, less moribund, and had significantly lower lung pathology than saline-treated mice. However, the viral burden in the lungs of MEDI3622-treated mice was significantly increased. Thus, ADAM17 appears to have a critical anti-viral role, but also may promote inflammatory damage. Since the inflammatory cascade is ultimately the reason for adverse outcomes in COVID-19 patients, there may be a therapeutic application for the MEDI3622 antibody.
Keywords: ADAM19; COVID-19; SARS-CoV-2; inflammation; lung; mouse model; virus.
Copyright © 2022 Hedges, Snyder, Robison, Grifka-Walk, Blackwell, Shepardson, Kominsky, Rynda-Apple, Walcheck and Jutila.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

