Arap1 loss causes retinal pigment epithelium phagocytic dysfunction and subsequent photoreceptor death
- PMID: 35758026
- PMCID: PMC9346516
- DOI: 10.1242/dmm.049343
Arap1 loss causes retinal pigment epithelium phagocytic dysfunction and subsequent photoreceptor death
Abstract
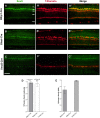
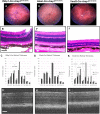
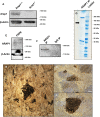
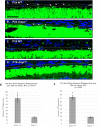
Retinitis pigmentosa (RP), a retinal degenerative disease, is the leading cause of heritable blindness. Previously, we described that Arap1-/- mice develop a similar pattern of photoreceptor degeneration. Arap1 is an Arf-directed GTPase-activating protein shown to modulate actin cytoskeletal dynamics. Curiously, Arap1 expression was detected in Müller glia and retinal pigment epithelium (RPE), but not the photoreceptors themselves. In this study, we generated conditional knockout mice for Müller glia/RPE, Müller glia and RPE via targeting Rlbp1, Glast and Vmd2 promoters, respectively, to drive Cre recombinase expression to knock out Arap1. Vmd2-Cre Arap1tm1c/tm1c and Rlbp1-Cre Arap1tm1c/tm1c mice, but not Glast-Cre Arap1tm1c/tm1c mice, recapitulated the phenotype originally observed in germline Arap1-/- mice. Mass spectrometry analysis of human ARAP1 co-immunoprecipitation identified candidate binding partners of ARAP1, revealing potential interactants involved in phagocytosis, cytoskeletal composition, intracellular trafficking and endocytosis. Quantification of outer segment phagocytosis in vivo demonstrated a clear phagocytic defect in Arap1-/- mice compared to Arap1+/+ controls. We conclude that Arap1 expression in RPE is necessary for photoreceptor survival due to its indispensable function in RPE phagocytosis. This article has an associated First Person interview with the first author of the paper.
Keywords: Arap1; Phagocytosis; Pigmentosa; RPE; Retinitis.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

