Immunogenicity and safety of SpikoGen®, an adjuvanted recombinant SARS-CoV-2 spike protein vaccine as a homologous and heterologous booster vaccination: A randomized placebo-controlled trial
- PMID: 35758850
- PMCID: PMC9349507
- DOI: 10.1111/imm.13540
Immunogenicity and safety of SpikoGen®, an adjuvanted recombinant SARS-CoV-2 spike protein vaccine as a homologous and heterologous booster vaccination: A randomized placebo-controlled trial
Abstract
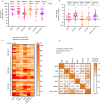
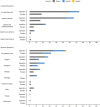
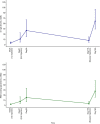
SpikoGen® is a subunit recombinant spike protein vaccine combined with Advax-CpG55.2™ adjuvant. This COVID-19 vaccine was shown to be safe, immunogenic and efficacious in previous clinical trials. This study aimed to assess the safety and immunogenicity of SpikoGen® vaccine as a homologous and heterologous booster vaccination. This double-blind and randomized placebo-controlled (5:1) trial was performed on 300 already vaccinated participants. SpikoGen® or saline placebo was administered as a booster dose to participants who had received a full two-dose COVID-19 vaccination course. Immunogenicity assessments were done 14 days after the booster dose with the primary immunogenicity outcome seroconversion rate of neutralizing antibodies. Safety outcomes included the incidence of solicited adverse events up to 7 days after the booster dose. SpikoGen® vaccine induced a robust humoral response both as a homologous and heterologous booster, when compared to the placebo. At Day 14, seroconversion of neutralizing antibodies was 76% (95% confidence interval [CI]: 69%-82%) in the SpikoGen® group versus 3% (95% CI: 0%-13%) in the placebo group. The most common local and systemic reported adverse events were injection site pain and fatigue. No serious adverse events were reported. The SpikoGen®-booster induced cross-neutralization of other SARS-CoV-2 variants. Irrespective of the primary vaccine course received, SpikoGen® vaccine showed promising effects as both a homologous and heterologous booster dose. This vaccine also had a good safety profile with no vaccine-associated serious adverse events. On the basis of these results, SpikoGen® vaccine has been approved as a booster dose.
Keywords: COVID-19; SARS-CoV-2; SpikoGen; booster; cross-neutralization.
© 2022 John Wiley & Sons Ltd.
Conflict of interest statement
NA, RSH, NF and SB are members of the Orchid Pharmed medical department which is in collaboration with CinnaGen company with respect to conducting clinical trials. KhR is in CinnaGen Medical Biotechnology Research Center. GA and NP are members of the Vaxine Pty Ltd. The remaining authors have no other relevant affiliations.
Figures


Similar articles
-
Immunogenicity and Safety of SpikoGen, an Adjuvanted Recombinant SARS-CoV-2 Spike Protein, as a Heterologous Third Booster Dose in Kidney Transplant Patients: A Single-arm Clinical Trial.Clin Ther. 2022 Dec;44(12):1566-1576. doi: 10.1016/j.clinthera.2022.10.002. Epub 2022 Oct 24. Clin Ther. 2022. PMID: 36402595 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of SpikoGen®, an Advax-CpG55.2-adjuvanted SARS-CoV-2 spike protein vaccine: a phase 2 randomized placebo-controlled trial in both seropositive and seronegative populations.Clin Microbiol Infect. 2022 Sep;28(9):1263-1271. doi: 10.1016/j.cmi.2022.04.004. Epub 2022 Apr 15. Clin Microbiol Infect. 2022. PMID: 35436611 Free PMC article. Clinical Trial.
-
Comparative immunogenicity and safety of SpikoGen®, a recombinant SARS-CoV-2 spike protein vaccine in children and young adults: An immuno-bridging clinical trial.Int Immunopharmacol. 2024 Jan 25;127:111436. doi: 10.1016/j.intimp.2023.111436. Epub 2023 Dec 25. Int Immunopharmacol. 2024. PMID: 38147778 Clinical Trial.
-
Clinical development of SpikoGen®, an Advax-CpG55.2 adjuvanted recombinant spike protein vaccine.Hum Vaccin Immunother. 2024 Dec 31;20(1):2363016. doi: 10.1080/21645515.2024.2363016. Epub 2024 Jun 5. Hum Vaccin Immunother. 2024. PMID: 38839044 Free PMC article. Review.
-
The effectiveness of the first dose COVID-19 booster vs. full vaccination to prevent SARS-CoV-2 infection and severe COVID-19 clinical event: a meta-analysis and systematic review of longitudinal studies.Front Public Health. 2023 Jun 1;11:1165611. doi: 10.3389/fpubh.2023.1165611. eCollection 2023. Front Public Health. 2023. PMID: 37325336 Free PMC article.
Cited by
-
Effects of age and gender on immunogenicity and reactogenicity of SpikoGen recombinant spike protein vaccine: a post-hoc analysis.Sci Rep. 2024 Sep 30;14(1):22631. doi: 10.1038/s41598-024-67945-3. Sci Rep. 2024. PMID: 39349494 Free PMC article.
-
Safety and immunogenicity of SCB-2019, an adjuvanted, recombinant SARS-CoV-2 trimeric S-protein subunit COVID-19 vaccine in healthy 12-17 year-old adolescents.Hum Vaccin Immunother. 2023 Dec 31;19(1):2206359. doi: 10.1080/21645515.2023.2206359. Epub 2023 May 25. Hum Vaccin Immunother. 2023. PMID: 37226504 Free PMC article. Clinical Trial.
-
Immunogenicity and safety of adjuvant-associated COVID-19 vaccines: A systematic review and meta-analysis of randomized controlled trials.Heliyon. 2023 Nov 28;9(12):e22858. doi: 10.1016/j.heliyon.2023.e22858. eCollection 2023 Dec. Heliyon. 2023. PMID: 38125524 Free PMC article. Review.
-
Dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) is a cellular receptor for delta inulin adjuvant.Immunol Cell Biol. 2024 Aug;102(7):593-604. doi: 10.1111/imcb.12774. Epub 2024 May 17. Immunol Cell Biol. 2024. PMID: 38757764 Free PMC article.
-
Ability of SpikoGen®, an Advax-CpG adjuvanted recombinant spike protein vaccine, to induce cross-neutralising antibodies against SARS-CoV-2 variants.Immunology. 2023 Oct;170(2):193-201. doi: 10.1111/imm.13661. Epub 2023 May 18. Immunology. 2023. PMID: 37199229 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

