A Targetable Myeloid Inflammatory State Governs Disease Recurrence in Clear-Cell Renal Cell Carcinoma
- PMID: 35758895
- PMCID: PMC9720541
- DOI: 10.1158/2159-8290.CD-21-0925
A Targetable Myeloid Inflammatory State Governs Disease Recurrence in Clear-Cell Renal Cell Carcinoma
Abstract
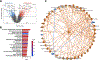
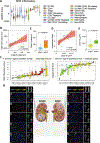
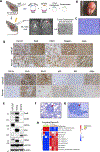
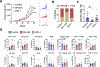
It is poorly understood how the tumor immune microenvironment influences disease recurrence in localized clear-cell renal cell carcinoma (ccRCC). Here we performed whole-transcriptomic profiling of 236 tumors from patients assigned to the placebo-only arm of a randomized, adjuvant clinical trial for high-risk localized ccRCC. Unbiased pathway analysis identified myeloid-derived IL6 as a key mediator. Furthermore, a novel myeloid gene signature strongly correlated with disease recurrence and overall survival on uni- and multivariate analyses and is linked to TP53 inactivation across multiple data sets. Strikingly, effector T-cell gene signatures, infiltration patterns, and exhaustion markers were not associated with disease recurrence. Targeting immunosuppressive myeloid inflammation with an adenosine A2A receptor antagonist in a novel, immunocompetent, Tp53-inactivated mouse model significantly reduced metastatic development. Our findings suggest that myeloid inflammation promotes disease recurrence in ccRCC and is targetable as well as provide a potential biomarker-based framework for the design of future immuno-oncology trials in ccRCC.
Significance: Improved understanding of factors that influence metastatic development in localized ccRCC is greatly needed to aid accurate prediction of disease recurrence, clinical decision-making, and future adjuvant clinical trial design. Our analysis implicates intratumoral myeloid inflammation as a key driver of metastasis in patients and a novel immunocompetent mouse model. This article is highlighted in the In This Issue feature, p. 2221.
©2022 American Association for Cancer Research.
Conflict of interest statement
Disclosures
T.A.C. is a co-founder of Gritstone Oncology and holds equity in An2H. T.A.C. acknowledges grant funding from Bristol-Myers Squibb, AstraZeneca, Illumina, Pfizer, An2H, and Eisai. T.A.C. has served as an advisor for Bristol-Myers, MedImmune, Squibb, Illumina, Eisai, AstraZeneca, and An2H. T.A.C. and V. L. hold ownership of intellectual property on using tumor mutation burden to predict immunotherapy response, with pending patent, which has been licensed to PGDx. R.J.M served in a consultancy or advisory role for Pfizer, Novartis, Merck, Genentech/Roche, Eisai and Exelixis, and received research funding from Bristol-Myers Squibb, Merck, Pfizer, Genentech/Roche, Eisai, Exelixis, and Novartis. M.H.V. reports honoraria from Novartis, consulting/advisory role for Alexion Pharmaceuticals, Bayer, Calithera Biosciences, Corvus Pharmaceuticals, Exelixis, Eisai, GlaxoSmithKline, Natera, Novartis and Pfizer, research funding from Pfizer, Bristol-Myers Squibb and Genentech/Roche, and travel, accommodations, and expenses from Eisai, Novartis and Takeda. J.M., M.M., A.R., and A.S. are employees of Novartis. A.A.H. has served in an advisory role for Merck. D.B.S. has consulted for/received honoraria from Pfizer, Loxo/Lilly Oncology, Vividion Therapeutics, FORE Therapeutics, Scorpion Therapeutics and BridgeBio. M.G. is a shareholder and an employee of Illumina Inc. The rest of the authors have no conflicts to disclose.
Figures



References
-
- Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang Y-H, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021;385:683–94. - PubMed
-
- Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: A stratification tool for prospective clinical trials. Cancer. 2003;97:1663–71. - PubMed
-
- Zisman A, Pantuck AJ, Wieder J, Chao DH, Dorey F, Said JW, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20:4559–66. - PubMed
-
- Morgan TM, Mehra R, Tiemeny P, Wolf JS, Wu S, Sangale Z, et al. A Multigene Signature Based on Cell Cycle Proliferation Improves Prediction of Mortality Within 5 Yr of Radical Nephrectomy for Renal Cell Carcinoma. Eur Urol. 2018;73:763–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

