TFEB-Mediated Lysosomal Restoration Alleviates High Glucose-Induced Cataracts Via Attenuating Oxidative Stress
- PMID: 35758908
- PMCID: PMC9248753
- DOI: 10.1167/iovs.63.6.26
TFEB-Mediated Lysosomal Restoration Alleviates High Glucose-Induced Cataracts Via Attenuating Oxidative Stress
Abstract
Purpose: Diabetic cataract (DC) is a visual disorder arising from diabetes mellitus (DM). Autophagy, a prosurvival intracellular process through lysosomal fusion and degradation, has been implicated in multiple diabetic complications. Herein, we performed in vivo and in vitro assays to explore the specific roles of the autophagy-lysosome pathway in DC.
Methods: Streptozotocin-induced DM and incubation in high glucose (HG) led to rat lens opacification. Protein Simple Wes, Western blot, and immunoassay were utilized to investigate autophagic changes in lens epithelial cells (LECs) and lens fiber cells (LFCs). RNA-sequencing (RNA-seq) was performed to explore genetic changes in the lenses of diabetic rats. Moreover, autophagy-lysosomal functions were examined using lysotracker, Western blot, and immunofluorescence analyses in HG-cultured primary rabbit LECs.
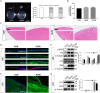
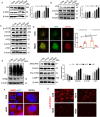
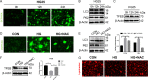
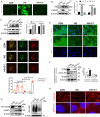
Results: First, DM and HG culture led to fibrotic LECs, swelling LFCs, and eventually cataracts. Further analysis showed aberrant autophagic degradation in LECs and LFCs during cataract formation. RNA-seq data revealed that the differentially expressed genes (DEGs) were enriched in the lysosome pathway. In primary LECs, HG treatment resulted in decreased transcription factor EB (TFEB) and cathepsin B (CTSB) activity, and increased lysosomal size and pH values. Moreover, TFEB-mediated dysfunctional lysosomes resulted from excessive oxidative stress in LECs under HG conditions. Furthermore, TFEB activation by curcumin analog C1 alleviated HG-induced cataracts through enhancing lysosome biogenesis and activating protective autophagy, thereby attenuating HG-mediated oxidative damage.
Conclusions: In summary, we first identified that ROS-TFEB-dependent lysosomal dysfunction contributed to autophagy blockage in HG-induced cataracts. Additionally, TFEB-mediated lysosomal restoration might be a promising therapeutic method for preventing and treating DC through mitigating oxidative stress.
Conflict of interest statement
Disclosure:
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

