A Guideline and Checklist for Initiating and Managing Clozapine Treatment in Patients with Treatment-Resistant Schizophrenia
- PMID: 35759211
- PMCID: PMC9243911
- DOI: 10.1007/s40263-022-00932-2
A Guideline and Checklist for Initiating and Managing Clozapine Treatment in Patients with Treatment-Resistant Schizophrenia
Erratum in
-
Correction: A Guideline and Checklist for Initiating and Managing Clozapine Treatment in Patients with Treatment-Resistant Schizophrenia.CNS Drugs. 2022 Sep;36(9):1015. doi: 10.1007/s40263-022-00946-w. CNS Drugs. 2022. PMID: 35976566 Free PMC article. No abstract available.
Abstract
Treatment-resistant schizophrenia (TRS) will affect about one in three patients with schizophrenia. Clozapine is the only treatment approved for TRS, and patients should be treated as soon as possible to improve their chances of achieving remission. Despite its effectiveness, concern over side effects, monitoring requirements, and inexperience with prescribing often result in long delays that can expose patients to unnecessary risks and compromise their chances of achieving favorable long-term outcomes. We critically reviewed the literature on clozapine use in TRS, focusing on guidelines, systematic reviews, and algorithms to identify strategies for improving clozapine safety and tolerability. Based on this, we have provided an overview of strategies to support early initiation of clozapine in patients with TRS based on the latest evidence and our clinical experience, and have summarized the key elements in a practical, evidence-based checklist for identifying and managing patients with TRS, with the aim of increasing confidence in prescribing and monitoring clozapine therapy.
© 2022. The Author(s).
Conflict of interest statement
Dr. Correll has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Axsome, Cardio Diagnostics, Compass, Damitsa, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Medscape, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Pharmabrain, Recordati, Relmada, Reviva, Rovi, Seqirus, Servier, SK Life Science, Sumitomo Dainippon, Sunovion, Supernus, Takeda, Teva, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Relmada, Reviva, Rovi, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and holds stock options for Cardio Diagnostics, Mindpax, and LB Pharma. Dr. Agid has been a consultant and/or advisor to or has received honoraria as follows: Advisory Board/Consultant: Janssen-Ortho (Johnson & Johnson); Otsuka; Lundbeck; Allergan/Abbvie; Speaker: Janssen-Ortho (Johnson & Johnson); Lundbeck, Otsuka, Mylan Pharmaceuticals; HLS Therapeutics; Research Contracts: Janssen-Ortho (Johnson & Johnson); Otsuka; Boehringer Ingelheim; Neurocrine Bioscience; DiaMentis. Prof. Crespo-Facorro has received honoraria as a consultant and/or advisor to Angelini, Janssen/J&J, Lundbeck, Otsuka and Viatris. Dr. de Bartolomeis has participated in advisory boards, lectures and unrestricted presentations sponsored by Trivia- Mylan. Dr. Fagiolini is /has been a consultant and/or a speaker and/or has received research grants from: Angelini, Apsen, Boehringer Ingelheim, Lundbeck, Janssen, Viatris, Otsuka, Recordati, Sanofi Aventis, Sunovion, Glaxo Smith Kline. Dr. Seppala is a part-time employee of Viatris Finland as a Consultant counselling doctors on clozapine-related problems. He has participated as advisor/speaker in meetings organized by Viatris, Recordati, and Janssen-Cilag. Dr. Howes is a part-time employee of H. Lundbeck A/S and has received investigator-initiated research funding from and/or participated in advisory/ speaker meetings organized by Angellini, Autifony, Biogen, Boehringer-Ingelheim, Eli Lilly, Heptares, Global Medical Education, Invicro, Jansenn, Lundbeck, Neurocrine, Otsuka, Sunovion, Rand, Recordati, Roche and Viatris/ Mylan.
Figures

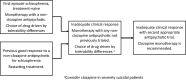



References
-
- GBD 2019 Mental Disorders Collaborators Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–150. doi: 10.1016/S2215-0366(21)00395-3. - DOI - PMC - PubMed

