Antiviral activity of triptolide on herpes simplex virus in vitro
- PMID: 35759241
- PMCID: PMC9208287
- DOI: 10.1002/iid3.667
Antiviral activity of triptolide on herpes simplex virus in vitro
Abstract
Background: Herpes simplex virus-type 1 (HSV-1) can cause diseases, especially amongst neonates and immunocompromised hosts. Hence, developing a novel anti-HSV-1 drug with low-level toxicity is vital. Triptolide (TP), a diterpenoid triepoxide is a natural product with range of bioactivity qualities.
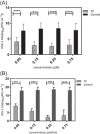
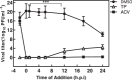
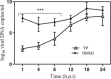
Methods: In this study, viral infection was assessed in different phases of the HSV-1 replication cycle on A549 cells, using various assays, such as adsorption inhibition assay, penetration inhibition assay, time-of-addition assay, and quantitative polymerase chain reaction (qPCR).
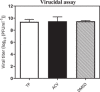
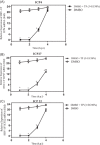
Results: The results indicate that TP can effectively inhibit HSV-1 infection in the lowest range of concentration. TP exhibited significant inhibitory effect on HSV-1 plaque formation, with 50% effective concentration (EC50) of 0.05 µM. Furthermore, the time-of-addition assay suggests that TP has viral inhibitory effects when it was added less than 8 h postinfection (h.p.i.). This result is further confirmed by decline in the expression viral immediate-early genes (ICP4, ICP22, and ICP27) in 6 h.p.i in the TP-treated group compared to the control group, evaluated by real-time qPCR. The Western blotting result was also consistent with the previous findings, which confirms that TP can positively affect ICP4 during HSV-1 infection.
Conclusions: The TP also showed antiviral activity against HSV-1. This dose-dependent activity is an indication of a particular cellular component, rather than cytotoxicity that has mediated its function. Finally, the result suggest a new approach for an effective treatment option of the HSV-1 infections.
Keywords: HSV-1; ICP4; antiviral; natural product; triptolide.
© 2022 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Fields BN. Fields' virology. Vol 1. Lippincott Williams & Wilkins; 2007.
-
- Smith JS, Robinson NJ. Age‐specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186(suppl 1):S3‐S28. - PubMed
-
- Kolokotronis A, Doumas S. Herpes simplex virus infection, with particular reference to the progression and complications of primary herpetic gingivostomatitis. Clin Microbiol Infect. 2006;12(3):202‐211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

