Comparative Effectiveness of Postdischarge Smoking Cessation Interventions for Hospital Patients: The Helping HAND 4 Randomized Clinical Trial
- PMID: 35759282
- PMCID: PMC9237801
- DOI: 10.1001/jamainternmed.2022.2300
Comparative Effectiveness of Postdischarge Smoking Cessation Interventions for Hospital Patients: The Helping HAND 4 Randomized Clinical Trial
Abstract
Importance: Smoking cessation interventions for hospitalized patients must continue after discharge to improve long-term tobacco abstinence. How health systems can best deliver postdischarge tobacco treatment is uncertain.
Objective: To determine if health system-based tobacco cessation treatment after hospital discharge produces more long-term tobacco abstinence than referral to a community-based quitline.
Design, setting, and participants: This randomized clinical trial was conducted September 2018 to November 2020 in 3 hospitals in Massachusetts, Pennsylvania, and Tennessee. Cigarette smokers admitted to a study hospital who received brief in-hospital tobacco treatment and wanted to quit smoking were recruited for participation and randomized for postdischarge treatment to health system-based Transitional Tobacco Care Management (TTCM) or electronic referral to a community-based quitline (QL). Both multicomponent interventions offered smoking cessation counseling and nicotine replacement therapy (NRT) for up to 3 months. Data were analyzed from February 1, 2021, to April 25, 2022.
Interventions: TTCM provided 8 weeks of NRT at discharge and 7 automated calls with a hospital-based counselor call-back option. The QL intervention sent referrals from the hospital electronic health record to the state quitline, which offered 5 counseling calls and an NRT sample.
Main outcomes and measures: The main outcome was biochemically verified past 7-day tobacco abstinence at 6 months. Self-reported point-prevalence and continuous tobacco abstinence and tobacco treatment utilization were assessed 1, 3, and 6 months after discharge.
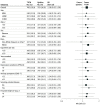
Results: A total of 1409 participants (mean [SD] age, 51.7 [12.6] years; 784 [55.6%] women; mean [SD] 16.4 [10.6] cigarettes/day) were recruited, including 706 randomized to TTCM and 703 randomized to QL. Participants were comparable at baseline, including 216 Black participants (15.3%), 82 Hispanic participants (5.8%), and 1089 White participants (77.3%). At 1 and 3 months after discharge, more TTCM participants than QL participants used cessation counseling (1 month: 245 participants [34.7%] vs 154 participants [21.9%]; 3 months: 248 participants [35.1%] vs 123 participants [17.5%]; P < .001) and pharmacotherapy (1 month: 455 participants [64.4%] vs 324 participants [46.1%]; 3 months: 367 participants [52.0%] vs 264 participants [37.6%]; P < .001). More TTCM than QL participants reported continuous abstinence for 3 months (RR, 1.30; 95% CI, 1.06-1.58) and point-prevalence abstinence at 1 month (RR, 1.22; 95% CI, 1.08-1.35) and 3 months (RR, 1.23; 95% CI, 1.09-1.37) but not at 6 months (RR, 1.14; 95% CI, 0.99-1.29). The primary outcome, biochemically verified point-prevalence abstinence at 6 months, was not statistically significantly different between groups (19.9% vs 16.9%; RR, 1.18; 95% CI, 0.92-1.50).
Conclusions and relevance: In this randomized clinical trial, biochemically verified tobacco abstinence rates were not significantly different between groups at the 6-month follow-up. However, the health system-based model was superior to the community-based quitline model throughout the 3 months of active treatment. A longer duration of postdischarge treatment may sustain the superiority of the health system-based model.
Trial registration: ClinicalTrials.gov Identifier: NCT03603496.
Conflict of interest statement
Figures

References
-
- US Department of Health and Human Services . Smoking cessation: a report of the Surgeon General—executive summary. Accessed May 20, 2022. https://www.hhs.gov/sites/default/files/2020-cessation-sgr-full-report.pdf - PubMed
-
- US Department of Health and Human Services . The Health Consequences of Smoking–50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention; 2014.
-
- National Center for Health Statistics . National Health Interview Survey. Accessed May 20, 2022. https://www.cdc.gov/nchs/nhis/2020nhis.htm
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

