Fecal Microbiota Transfer Attenuates Gut Dysbiosis and Functional Deficits After Traumatic Brain Injury
- PMID: 35759305
- PMCID: PMC10341382
- DOI: 10.1097/SHK.0000000000001934
Fecal Microbiota Transfer Attenuates Gut Dysbiosis and Functional Deficits After Traumatic Brain Injury
Abstract
Background: Traumatic brain injury (TBI) is an underrecognized public health threat. Survivors of TBI often suffer long-term neurocognitive deficits leading to the progressive onset of neurodegenerative disease. Recent data suggests that the gut-brain axis is complicit in this process. However, no study has specifically addressed whether fecal microbiota transfer (FMT) attenuates neurologic deficits after TBI.
Hypothesis: We hypothesized that fecal microbiota transfer would attenuate neurocognitive, anatomic, and pathologic deficits after TBI.
Methods: C57Bl/6 mice were subjected to severe TBI (n = 20) or sham-injury (n = 20) via an open-head controlled cortical impact. Post-injury, this cohort of mice underwent weekly oral gavage with a slurry of healthy mouse stool or vehicle alone beginning 1 h post-TBI followed by behavioral testing and neuropathologic analysis. 16S ribosomal RNA sequencing of fecal samples was performed to characterize gut microbial community structure pre- and post-injury. Zero maze and open field testing were used to evaluate post-traumatic anxiety, exploratory behavior, and generalized activity. 3D, contrast enhanced, magnetic resonance imaging was used to determine differences in cortical volume loss and white matter connectivity. Prior to euthanasia, brains were harvested for neuropathologic analysis.
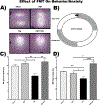
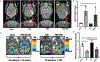
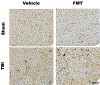
Results: Fecal microbiome analysis revealed a large variance between TBI, and sham animals treated with vehicle, while FMT treated TBI mice had restoration of gut dysbiosis back to levels of control mice. Neurocognitive testing demonstrated a rescue of normal anxiety-like and exploratory behavior in TBI mice treated with FMT. FMT treated TBI mice spent a greater percentage of time (22%, P = 0.0001) in the center regions of the Open Field as compared to vehicle treated TBI mice (13%). Vehicle-treated TBI animals also spent less time (19%) in the open areas of zero maze than FMT treated TBI mice (30%, P = 0.0001). Comparing in TBI mice treated with FMT, MRI demonstrated a marked attenuation in ventriculomegaly (P < 0.002) and a significant change in fractional anisotropy (i.e., loss of white matter connectivity) (P < 0.0001). Histologic analysis of brain sections revealed a FMT- injury dependent interaction in the microglia/macrophage-specific ionized calcium-binding protein, Iba1 (P = 0.002).
Conclusion: These data suggest that restoring a pre-injury gut microbial community structure may be a promising therapeutic intervention after TBI.
Copyright © 2022 by the Shock Society.
Conflict of interest statement
The authors report no conflict(s) of interest.
Figures
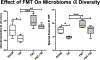
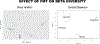

References
-
- Nittayasoot N, Peterson AB, Thammawijaya P, Parker EM, Sathawornwiwat A, Boonthanapat N, Chantian T, Voradetwitaya L, Jiraphongsa C, Sagarasaeranee O, et al.: Evaluation of a hospital-based injury surveillance system for monitoring road traffic deaths in Phuket, Thailand. Traffic Inj Prev 20(4):365–371, 2019. - PMC - PubMed
-
- Faul M, Coronado V. Chapter 1: Epidemiology of traumatic brain injury In: Grafman J, Salazar AM, eds. Vol. 127. Elsevier, 2015, 3–13. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

