Recombinant vesicular stomatitis vaccine against Nipah virus has a favorable safety profile: Model for assessment of live vaccines with neurotropic potential
- PMID: 35759511
- PMCID: PMC9269911
- DOI: 10.1371/journal.ppat.1010658
Recombinant vesicular stomatitis vaccine against Nipah virus has a favorable safety profile: Model for assessment of live vaccines with neurotropic potential
Abstract
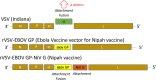
Nipah virus (NiV) disease is a bat-borne zoonosis responsible for outbreaks with high lethality and is a priority for vaccine development. With funding from the Coalition of Epidemic Preparedness Innovations (CEPI), we are developing a chimeric vaccine (PHV02) composed of recombinant vesicular stomatitis virus (VSV) expressing the envelope glycoproteins of both Ebola virus (EBOV) and NiV. The EBOV glycoprotein (GP) mediates fusion and viral entry and the NiV attachment glycoprotein (G) is a ligand for cell receptors, and stimulates neutralizing antibody, the putative mediator of protection against NiV. PHV02 is identical in construction to the registered Ebola vaccine (Ervebo) with the addition of the NiV G gene. NiV ephrin B2 and B3 receptors are expressed on neural cells and the wild-type NiV is neurotropic and causes encephalitis in affected patients. It was therefore important to assess whether the NiV G alters tropism of the rVSV vector and serves as a virulence factor. PHV02 was fully attenuated in adult hamsters inoculated by the intramuscular (IM) route, whereas parental wild-type VSV was 100% lethal. Two rodent models (mice, hamsters) were infected by the intracerebral (IC) route with graded doses of PHV02. Comparator active controls in various experiments included rVSV-EBOV (representative of Ebola vaccine) and yellow fever (YF) 17DD commercial vaccine. These studies showed PHV02 to be more neurovirulent than both rVSV-EBOV and YF 17DD in infant animals. PHV02 was lethal for adult hamsters inoculated IC but not for adult mice. In contrast YF 17DD retained virulence for adult mice inoculated IC but was not virulent for adult hamsters. Because of the inconsistency of neurovirulence patterns in the rodent models, a monkey neurovirulence test (MNVT) was performed, using YF 17DD as the active comparator because it has a well-established profile of quantifiable microscopic changes in brain centers and a known reporting rate of neurotropic adverse events in humans. In the MNVT PHV02 was significantly less neurovirulent than the YF 17DD vaccine reference control, indicating that the vaccine will have an acceptable safety profile for humans. The findings are important because they illustrate the complexities of phenotypic assessment of novel viral vectors with tissue tropisms determined by transgenic proteins, and because it is unprecedented to use a heterologous comparator virus (YF vaccine) in a regulatory-enabling study. This approach may have value in future studies of other novel viral vectors.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: BLS is employed by Q2 Solutions and MDW by BioReliance. WTA is employed by VirtuStat, YVC is employed by AmplifyBio, and YVC, EHM, and RJH are employees of Battelle. JF, RN, and KS are employees of, and TPG is a consultant to Public Health Vaccines LLC (PHV), which holds licenses to patents covering aspects of the rVSV-Nipah technology from Public Health Canada and the (US) National Institutes of Health and is engaged in development of the PHV02 vaccine. TPM and LT are members of the PHV Board of Directors. TPM and LT are employees and shareholders of Crozet BioPharma LLC. JF, RN, and KS are also shareholders in Crozet
Figures
References
-
- Singh RK, Dhama K, Chakraborty S, Tiwari R, Natesan S, Khandia R, et al.. Nipah virus: epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies - a comprehensive review. Vet Q. 2019. Dec;39(1):26–55. doi: 10.1080/01652176.2019.1580827 ; PMCID: PMC6830995. - DOI - PMC - PubMed
-
- DeBuysscher BL, Scott D, Marzi A, Prescott J, Feldmann H. Single-dose live-attenuated Nipah virus vaccines confer complete protection by eliciting antibodies directed against surface glycoproteins. Vaccine. 2014. May 7;32(22):2637–44. doi: 10.1016/j.vaccine.2014.02.087 Epub 2014 Mar 12. ; PMCID: PMC4829066. - DOI - PMC - PubMed
-
- Prescott J, DeBuysscher BL, Feldmann F, Gardner DJ, Haddock E, Martellaro C, et al.. Single-dose live-attenuated vesicular stomatitis virus-based vaccine protects African green monkeys from Nipah virus disease. Vaccine. 2015. Jun 4;33(24):2823–9. doi: 10.1016/j.vaccine.2015.03.089 Epub 2015 Apr 10. ; PMCID: PMC4433813. - DOI - PMC - PubMed
-
- Monath TP, Fast PE, Modjarrad K, Clarke DK, Martin BK, Fusco J, et al.. rVSVΔG-ZEBOV-GP (also designated V920) recombinant vesicular stomatitis virus pseudotyped with Ebola Zaire Glycoprotein: Standardized template with key considerations for a risk/benefit assessment. Vaccine X. 2019. Jan 29;1:100009. doi: 10.1016/j.jvacx.2019.100009 ; PMCID: PMC6668225. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

