PHEV infection: A promising model of betacoronavirus-associated neurological and olfactory dysfunction
- PMID: 35759516
- PMCID: PMC9282652
- DOI: 10.1371/journal.ppat.1010667
PHEV infection: A promising model of betacoronavirus-associated neurological and olfactory dysfunction
Abstract
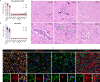
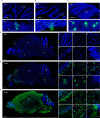
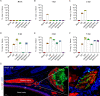
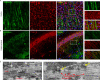
Porcine hemagglutinating encephalomyelitis virus (PHEV) is a highly neurotropic coronavirus belonging to the genus Betacoronavirus. Similar to pathogenic coronaviruses to which humans are susceptible, such as SARS-CoV-2, PHEV is transmitted primarily through respiratory droplets and close contact, entering the central nervous system (CNS) from the peripheral nerves at the site of initial infection. However, the neuroinvasion route of PHEV are poorly understood. Here, we found that BALB/c mice are susceptible to intranasal PHEV infection and showed distinct neurological manifestations. The behavioral study and histopathological examination revealed that PHEV attacks neurons in the CNS and causes significant smell and taste dysfunction in mice. By tracking neuroinvasion, we identified that PHEV invades the CNS via the olfactory nerve and trigeminal nerve located in the nasal cavity, and olfactory sensory neurons (OSNs) were susceptible to viral infection. Immunofluorescence staining and ultrastructural observations revealed that viral materials traveling along axons, suggesting axonal transport may engage in rapid viral transmission in the CNS. Moreover, viral replication in the olfactory system and CNS is associated with inflammatory and immune responses, tissue disorganization and dysfunction. Overall, we proposed that PHEV may serve as a potential prototype for elucidating the pathogenesis of coronavirus-associated neurological complications and olfactory and taste disorders.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

