Delta-like ligand 3-targeted radioimmunotherapy for neuroendocrine prostate cancer
- PMID: 35759660
- PMCID: PMC9271187
- DOI: 10.1073/pnas.2203820119
Delta-like ligand 3-targeted radioimmunotherapy for neuroendocrine prostate cancer
Abstract
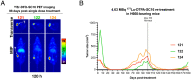
Neuroendocrine prostate cancer (NEPC) is a lethal subtype of prostate cancer with limited meaningful treatment options. NEPC lesions uniquely express delta-like ligand 3 (DLL3) on their cell surface. Taking advantage of DLL3 overexpression, we developed and evaluated lutetium-177 (177Lu)-labeled DLL3-targeting antibody SC16 (177Lu-DTPA-SC16) as a treatment for NEPC. SC16 was functionalized with DTPA-CHX-A" chelator and radiolabeled with 177Lu to produce 177Lu-DTPA-SC16. Specificity and selectivity of 177Lu-DTPA-SC16 were evaluated in vitro and in vivo using NCI-H660 (NEPC, DLL3-positive) and DU145 (adenocarcinoma, DLL3-negative) cells and xenografts. Dose-dependent treatment efficacy and specificity of 177Lu-DTPA-SC16 radionuclide therapy were evaluated in H660 and DU145 xenograft-bearing mice. Safety of the agent was assessed by monitoring hematologic parameters. 177Lu-DTPA-SC16 showed high tumor uptake and specificity in H660 xenografts, with minimal uptake in DU145 xenografts. At all three tested doses of 177Lu-DTPA-SC16 (4.63, 9.25, and 27.75 MBq/mouse), complete responses were observed in H660-bearing mice; 9.25 and 27.75 MBq/mouse doses were curative. Even the lowest tested dose proved curative in five (63%) of eight mice, and recurring tumors could be successfully re-treated at the same dose to achieve complete responses. In DU145 xenografts, 177Lu-DTPA-SC16 therapy did not inhibit tumor growth. Platelets and hematocrit transiently dropped, reaching nadir at 2 to 3 wk. This was out of range only in the highest-dose cohort and quickly recovered to normal range by week 4. Weight loss was observed only in the highest-dose cohort. Therefore, our data demonstrate that 177Lu-DTPA-SC16 is a potent and safe radioimmunotherapeutic agent for testing in humans with NEPC.
Keywords: DLL3; lutetium-177; neuroendocrine prostate cancer; radioimmunotherapy.
Conflict of interest statement
Competing interest statement: C.M.R. has consulted regarding oncology drug development with AbbVie, Amgen, AstraZeneca, Daiichi Sankyo, Epizyme, Genentech/Roche, Ipsen, Jazz, Kowa, Lilly, Merck, and Syros and serves on the scientific advisory boards of Bridge Medicines, Earli, and Harpoon Therapeutics. J.S.L. is cofounder and holds equity in pHLIP, Inc., and Sharp RTx, Inc. He is coinventor on licensed technology to Elucida Oncology, Samus Therapeutics, Diaprost, Macrocyclics, and Daiichi Sankyo. J.S.L. receives compensation for advisory roles from Clarity Pharmaceuticals, Varian Medical Systems, Evergreen Theragnostics, Telix Pharmaceuticals, Curie Therapeutics, Boxer, Earli, and TPG Capital. C.M.R., J.S.L., and J.T.P. have licensed intellectual property and have received royalty payments for DLL3 antibodies not used in this study. L.B. has served as an unremunerated consultant/speaker for AAA-Novartis, ITM, Iba, Clovis Oncology, and MTTI and has received a research grant from AAA-Novartis. M.J.M. is an uncompensated advisor to Novartis, Bayer, Lantheus, and Janssen and is a compensated consultant for ORIC, Curium, Athenex, AstraZeneca, and Amgen. M.J.M.’s institution receives funding for the conduct of clinical trials from Bayer, Corcept, Janssen, and Celgene.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

