Antibody Response to COVID-19 mRNA Vaccine in Patients With Lung Cancer After Primary Immunization and Booster: Reactivity to the SARS-CoV-2 WT Virus and Omicron Variant
- PMID: 35759727
- PMCID: PMC9671759
- DOI: 10.1200/JCO.21.02986
Antibody Response to COVID-19 mRNA Vaccine in Patients With Lung Cancer After Primary Immunization and Booster: Reactivity to the SARS-CoV-2 WT Virus and Omicron Variant
Abstract
Purpose: To examine COVID-19 mRNA vaccine-induced binding and neutralizing antibody responses in patients with non-small-cell lung cancer (NSCLC) to SARS-CoV-2 614D (wild type [WT]) strain and variants of concern after the primary 2-dose and booster vaccination.
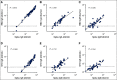
Methods: Eighty-two patients with NSCLC and 53 healthy volunteers who received SARS-CoV-2 mRNA vaccines were included in the study. Blood was collected longitudinally, and SARS-CoV-2-specific binding and neutralizing antibody responses were evaluated by Meso Scale Discovery assay and live virus Focus Reduction Neutralization Assay, respectively.
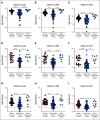
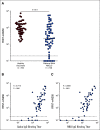
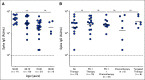
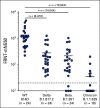
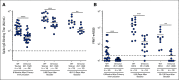
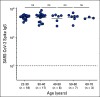
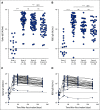
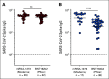
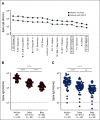
Results: A majority of patients with NSCLC generated binding and neutralizing antibody titers comparable with the healthy vaccinees after mRNA vaccination, but a subset of patients with NSCLC (25%) made poor responses, resulting in overall lower (six- to seven-fold) titers compared with the healthy cohort (P = < .0001). Although patients age > 70 years had lower immunoglobulin G titers (P = < .01), patients receiving programmed death-1 monotherapy, chemotherapy, or a combination of both did not have a significant impact on the antibody response. Neutralizing antibody titers to the B.1.617.2 (Delta), B.1.351 (Beta), and in particular, B.1.1.529 (Omicron) variants were significantly lower (P = < .0001) compared with the 614D (WT) strain. Booster vaccination led to a significant increase (P = .0001) in the binding and neutralizing antibody titers to the WT and Omicron variant. However, 2-4 months after the booster, we observed a five- to seven-fold decrease in neutralizing titers to WT and Omicron viruses.
Conclusion: A subset of patients with NSCLC responded poorly to the SARS-CoV-2 mRNA vaccination and had low neutralizing antibodies to the B.1.1.529 Omicron variant. Booster vaccination increased binding and neutralizing antibody titers to Omicron, but antibody titers declined after 3 months. These data highlight the concern for patients with cancer given the rapid spread of SARS-CoV-2 Omicron variant.
Conflict of interest statement
Antibody Response to COVID-19 mRNA Vaccine in Patients With Lung Cancer After Primary Immunization and Booster: Reactivity to SARS-CoV-2 WT Virus and Omicron Variant
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
Update of
-
Antibody response to SARS-CoV-2 mRNA vaccine in lung cancer patients: Reactivity to vaccine antigen and variants of concern.medRxiv [Preprint]. 2022 Jan 23:2022.01.03.22268599. doi: 10.1101/2022.01.03.22268599. medRxiv. 2022. Update in: J Clin Oncol. 2022 Nov 20;40(33):3808-3816. doi: 10.1200/JCO.21.02986. PMID: 35018383 Free PMC article. Updated. Preprint.
Comment in
-
Longitudinal Analyses of COVID-19 Vaccination in Patients With Lung Cancer: Antibody Responses and Variant-Specific Neutralization.J Clin Oncol. 2022 Nov 20;40(33):3787-3789. doi: 10.1200/JCO.22.01136. Epub 2022 Jun 27. J Clin Oncol. 2022. PMID: 35759731 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

