Aberrant expansion of spontaneous splenic germinal centers induced by hallmark genetic lesions of aggressive lymphoma
- PMID: 35759728
- PMCID: PMC9461474
- DOI: 10.1182/blood.2022015926
Aberrant expansion of spontaneous splenic germinal centers induced by hallmark genetic lesions of aggressive lymphoma
Abstract
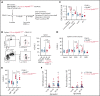
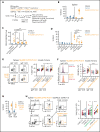
Unique molecular vulnerabilities have been identified in the aggressive MCD/C5 genetic subclass of diffuse large B-cell lymphoma (DLBCL). However, the premalignant cell-of-origin exhibiting MCD-like dependencies remains elusive. In this study, we examined animals carrying up to 4 hallmark genetic lesions found in MCD consisting of gain-of-function mutations in Myd88 and Cd79b, loss of Prdm1, and overexpression of BCL2. We discovered that expression of combinations of these alleles in vivo promoted a cell-intrinsic accumulation of B cells in spontaneous splenic germinal centers (GCs). As with MCD, these premalignant B cells were enriched for B-cell receptors (BCRs) with evidence of self-reactivity, displayed a de novo dependence on Tlr9, and were more sensitive to inhibition of Bruton's tyrosine kinase. Mutant spontaneous splenic GC B cells (GCB) showed increased proliferation and IRF4 expression. Mice carrying all 4 genetic lesions showed a >50-fold expansion of spontaneous splenic GCs exhibiting aberrant histologic features with a dark zone immunophenotype and went on to develop DLBCL in the spleen with age. Thus, by combining multiple hallmark genetic alterations associated with MCD, our study identifies aberrant spontaneous splenic GCBs as a likely cell-of-origin for this aggressive genetic subtype of lymphoma.
© 2022 by The American Society of Hematology.
Figures






Comment in
-
MCD-DLBCL arises from germinal center B cells.Blood. 2022 Sep 8;140(10):1058-1059. doi: 10.1182/blood.2022017534. Blood. 2022. PMID: 36074533 Free PMC article. No abstract available.
References
-
- Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503-511. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

