Response of COVID-19 vaccination in multiple sclerosis patients following disease-modifying therapies: A meta-analysis
- PMID: 35759920
- PMCID: PMC9230320
- DOI: 10.1016/j.ebiom.2022.104102
Response of COVID-19 vaccination in multiple sclerosis patients following disease-modifying therapies: A meta-analysis
Abstract
Background: COVID-19 vaccination is recommended for patients with multiple sclerosis (pwMS), while disease-modifying therapies (DMTs) may influence the efficacy of SARS-CoV-2 vaccines in this population. Thus, we conducted a meta-analysis to evaluate the impact of DMTs on immune response to COVID-19 vaccines in pwMS.
Methods: Literature search from December 1, 2019 to March 31, 2022 was performed in PubMed, MedRxiv, Embase and Cochrane Library. The risk of impaired response to vaccination in pwMS receiving DMTs was estimated in odds ratios (ORs) using random-effects method.
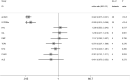
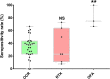
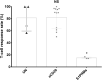
Findings: A total of 48 studies comprising 6860 pwMS were included. Overall, pwMS with anti-CD20 (OR=0.02, 95% CI: 0.01-0.03) and sphingosine-1-phosphate receptor modulator (S1PRM) (OR=0.03, 95% CI: 0.01-0.06) treatments had attenuated serologic response after full vaccination compared with those without DMTs. Additionally, pwMS vaccinated within six months since last anti-CD20 therapy were at significantly higher risk of blunted response compared with those receiving anti-CD20 therapy more than six months prior to vaccination (P = 0.001). We found no significant associations between other treatments (including IFN-β, GA, DMF, TERI, NTZ, CLAD, and ALE) and humoral response to SARS-CoV-2 vaccines in pwMS. As for T-cell response, no significant difference was found between pwMS on anti-CD20 and those without DMTs after vaccination, while S1PRM was marginally associated with impaired cellular response (P = 0.03).
Interpretation: Our findings suggested that routine serological monitoring may be required for pwMS on anti-CD20 and S1PRMs after SARS-CoV-2 vaccination and highlighted the benefits of a booster dose. The effect of cellular response and optimal interval from last anti-CD20 treatment to vaccination should be further addressed.
Funding: This study was supported by Natural Science Foundation of Shanghai (21ZR1433000).
Keywords: COVID-19 vaccines; Disease-modifying therapies; Immune response; Meta-analysis; Multiple sclerosis.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

