When to test for COVID-19 using real-time reverse transcriptase polymerase chain reaction: a systematic review
- PMID: 35760382
- PMCID: PMC9233872
- DOI: 10.1016/j.ijid.2022.06.037
When to test for COVID-19 using real-time reverse transcriptase polymerase chain reaction: a systematic review
Abstract
Objectives: The aim of this study was to evaluate the time in days between symptom onset and first positive real-time reverse transcriptase polymerase chain reaction (RT-PCR) result for COVID-19.
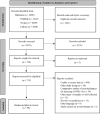
Methods: This systematic review was conducted in the MEDLINE (PubMed), Embase, and Scopus databases using the following descriptors: "COVID-19", "SARS-CoV-2", "coronavirus", "RT-PCR", "real time PCR", and "diagnosis".
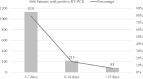
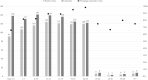
Results: The included studies were conducted in 31 different countries and reported on a total of 6831 patients. The median age of the participants was 49.95 years. The three most common symptoms were fever, cough, and dyspnea, which affected 4012 (58.68%), 3192 (46.69%), and 2009 patients (29.38%), respectively. Among the 90 included studies, 13 were prospective cohorts, 15 were retrospective cohorts, 36 were case reports, 20 were case series, and six were cross-sectional studies. The overall mean time between symptom onset and positive test result was 6.72 days. Fourteen articles were analyzed separately for the temporal profile of RT-PCR test results; the best performance was on days 22-24, when 98% of test results were positive.
Conclusion: These findings corroborate the RT-PCR COVID-19 testing practices of some health units. In addition, the most frequently described symptoms of these patients can be considered the initial symptoms of infection and used in decision-making about RT-PCR testing.
Keywords: Coronavirus(,) COVID-19; RT-PCR; Real-time reverse transcriptase polymerase chain reaction; SARS-CoV-2; Systematic review.
Copyright © 2022. Published by Elsevier Ltd.
Figures
Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Thoracic imaging tests for the diagnosis of COVID-19.Cochrane Database Syst Rev. 2022 May 16;5(5):CD013639. doi: 10.1002/14651858.CD013639.pub5. Cochrane Database Syst Rev. 2022. PMID: 35575286 Free PMC article.
-
Diagnostic test accuracy and cost-effectiveness of tests for codeletion of chromosomal arms 1p and 19q in people with glioma.Cochrane Database Syst Rev. 2022 Mar 2;3(3):CD013387. doi: 10.1002/14651858.CD013387.pub2. Cochrane Database Syst Rev. 2022. PMID: 35233774 Free PMC article.
Cited by
-
Radiation Dose Estimation and Lifetime Attributable Risk of Radiation-Induced Cancer from Chest CT in COVID-19 Patients.Ir J Med Sci. 2025 Aug 8. doi: 10.1007/s11845-025-04046-8. Online ahead of print. Ir J Med Sci. 2025. PMID: 40779137
-
The long-term health outcomes, pathophysiological mechanisms and multidisciplinary management of long COVID.Signal Transduct Target Ther. 2023 Nov 1;8(1):416. doi: 10.1038/s41392-023-01640-z. Signal Transduct Target Ther. 2023. PMID: 37907497 Free PMC article. Review.
-
Test allocation based on risk of infection from first and second order contact tracing.PLoS One. 2025 Apr 7;20(4):e0320291. doi: 10.1371/journal.pone.0320291. eCollection 2025. PLoS One. 2025. PMID: 40193399 Free PMC article.
-
Development and Characterization of Phage Display-Derived Monoclonal Antibodies to the S2 Domain of Spike Proteins of Wild-Type SARS-CoV-2 and Multiple Variants.Viruses. 2023 Jan 6;15(1):174. doi: 10.3390/v15010174. Viruses. 2023. PMID: 36680213 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

