Selective Ablation of Sod2 in Astrocytes Induces Sex-Specific Effects on Cognitive Function, d-Serine Availability, and Astrogliosis
- PMID: 35760531
- PMCID: PMC9351643
- DOI: 10.1523/JNEUROSCI.2543-21.2022
Selective Ablation of Sod2 in Astrocytes Induces Sex-Specific Effects on Cognitive Function, d-Serine Availability, and Astrogliosis
Abstract
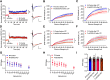
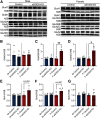
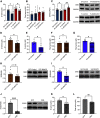
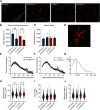
Cognitive decline is a debilitating aspect of aging and neurodegenerative diseases such as Alzheimer's disease are closely associated with mitochondrial dysfunction, increased reactive oxygen species, neuroinflammation, and astrogliosis. This study investigated the effects of decreased mitochondrial antioxidant response specifically in astrocytes on cognitive performance and neuronal function in C57BL/6J mice using a tamoxifen-inducible astrocyte-specific knockout of manganese superoxide dismutase (aSOD2-KO), a mitochondrial matrix antioxidant that detoxifies superoxide generated during mitochondrial respiration. We reduced astrocyte SOD2 levels in male and female mice at 11-12 months of age and tested in an automated home cage (PhenoTyper) apparatus for diurnal patterns, spatial learning, and memory function at 15 months of age. aSOD2-KO impaired hippocampal-dependent spatial working memory and decreased cognitive flexibility in the reversal phase of the testing paradigm in males. Female aSOD2-KO showed no learning and memory deficits compared with age-matched controls despite significant reduction in hippocampal SOD2 expression. aSOD2-KO males further showed decreased hippocampal long-term potentiation, but paired-pulse facilitation was unaffected. Levels of d-serine, an NMDA receptor coagonist, were also reduced in aSOD2-KO mice, but female knockouts showed a compensatory increase in serine racemase expression. Furthermore, aSOD2-KO mice demonstrated increased density of astrocytes, indicative of astrogliosis, in the hippocampus compared with age-matched controls. These data demonstrate that reduction in mitochondrial antioxidant stress response in astrocytes recapitulates age-related deficits in cognitive function, d-serine availability, and astrogliosis. Therefore, improving astrocyte mitochondrial homeostasis may provide a therapeutic target for intervention for cognitive impairment in aging.SIGNIFICANCE STATEMENT Diminished antioxidant response is associated with increased astrogliosis in aging and in Alzheimer's disease. Manganese superoxide dismutase (SOD2) is an antioxidant in the mitochondrial matrix that detoxifies superoxide and maintains mitochondrial homeostasis. We show that astrocytic ablation of SOD2 impairs hippocampal-dependent plasticity in spatial working memory, reduces long-term potentiation of hippocampal neurons and levels of the neuromodulator d-serine, and increases astrogliosis, consistent with defects in advanced aging and Alzheimer's disease. Our data provide strong evidence for sex-specific effects of astrocytic SOD2 functions in age-related cognitive dysfunction.
Keywords: SOD2; aging; astrogliosis; cognitive function; d-serine; long-term potentiation.
Copyright © 2022 the authors.
Figures


References
-
- Bourgognon JM, Spiers JG, Robinson SW, Scheiblich H, Glynn P, Ortori C, Bradley SJ, Tobin AB, Steinert JR (2021) Inhibition of neuroinflammatory nitric oxide signaling suppresses glycation and prevents neuronal dysfunction in mouse prion disease. Proc Natl Acad Sci U|S|A 118:e2009579118. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
