Self-Examination Low-Cost Full-Field Optical Coherence Tomography (SELFF-OCT) for neovascular age-related macular degeneration: a cross-sectional diagnostic accuracy study
- PMID: 35760534
- PMCID: PMC9237881
- DOI: 10.1136/bmjopen-2021-055082
Self-Examination Low-Cost Full-Field Optical Coherence Tomography (SELFF-OCT) for neovascular age-related macular degeneration: a cross-sectional diagnostic accuracy study
Abstract
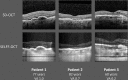
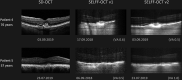
Objectives: Self-Examination Low-Cost Full-Field Optical Coherence Tomography (SELFF-OCT) is a novel OCT technology that was specifically designed for home monitoring of neovascular age-related macular degeneration (AMD). First clinical findings have been reported before. This trial investigates an improved prototype for patients with AMD and focusses on device operability and diagnostic accuracy compared with established spectral-domain OCT (SD-OCT).
Design: Prospective single-arm diagnostic accuracy study.
Setting: Tertiary care centre (University Eye Clinic).
Participants: 46 patients with age-related macular degeneration.
Interventions: Patients received short training in device handling and then performed multiple self-scans with the SELFF-OCT according to a predefined protocol. Additionally, all eyes were examined with standard SD-OCT, performed by medical personnel. All images were graded by at least 2 masked investigators in a reading centre.
Primary outcome measure: Rate of successful self-measurements.
Secondary outcome measures: Sensitivity and specificity of SELFF-OCT versus SD-OCT for different biomarkers and necessity for antivascular endothelial growth factor (anti-VEGF) treatment.
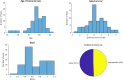
Results: In 86% of all examined eyes, OCT self-acquisition resulted in interpretable retinal OCT volume scans. In these patients, the sensitivity for detection of anti-VEGF treatment necessity was 0.94 (95% CI 0.79 to 0.99) and specificity 0.95 (95% CI 0.82 to 0.99).
Conclusions: SELFF-OCT was used successfully for retinal self-examination in most patients, and it could become a valuable tool for retinal home monitoring in the future. Improvements are in progress to reduce device size and to improve handling, image quality and success rates.
Trial registration number: DRKS00013755, CIV-17-12-022384.
Keywords: medical retina; ophthalmology; vetreoretinal.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: HS, PK, MMü and GH hold a patent related to SELFF-OCT. None of the other authors has any conflicts of interest to disclose.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
