POLYRETINA restores light responses in vivo in blind Göttingen minipigs
- PMID: 35760775
- PMCID: PMC9237028
- DOI: 10.1038/s41467-022-31180-z
POLYRETINA restores light responses in vivo in blind Göttingen minipigs
Abstract
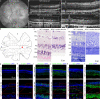
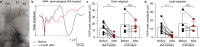
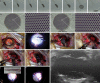
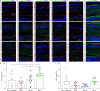
Retinal prostheses hold the potential for artificial vision in blind people affected by incurable diseases of the outer retinal layer. Available technologies provide only a small field of view: a significant limitation for totally blind people. To overcome this problem, we recently proposed a large and high-density photovoltaic epiretinal device, known as POLYRETINA. Here, we report the in vivo assessment of POLYRETINA. First, we characterise a model of chemically-induced blindness in Göttingen minipigs. Then, we develop and test a minimally invasive injection procedure to insert the large epiretinal implant into the eye. Last, we show that POLYRETINA restores light-evoked cortical responses in blind animals at safe irradiance levels. These results indicate that POLYRETINA holds the potential for artificial vision in totally blind patients affected by retinitis pigmentosa.
© 2022. The Author(s).
Conflict of interest statement
D.G. and M.J.I.A.L. are authors of a patent covering the POLYRETINA device [Applicants: École Polytechnique Fédérale de Lausanne; Inventors: Ghezzi Diego, Airaghi Leccardi Marta Jole Ildelfonsa and Ferlauto Laura; Application number: WO2018177547A1]. D.G., C.-H.V. and T.J.W. are authors of a patent covering the injector [Applicants: École Polytechnique Fédérale de Lausanne and Fondation Asile des Aveugles; Inventors: Ghezzi Diego, Wolfensberger Thomas and Vila Charles-Henri; Application number: WO2020229683A1]. The remaining authors declare no competing interests.
Figures




References
-
- Zrenner E. Fighting blindness with microelectronics. Sci. Transl. Med. 2013;5:210ps16–210ps16. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

