Blood-based biomarkers of antidepressant response to ketamine and esketamine: A systematic review and meta-analysis
- PMID: 35760879
- PMCID: PMC9933928
- DOI: 10.1038/s41380-022-01652-1
Blood-based biomarkers of antidepressant response to ketamine and esketamine: A systematic review and meta-analysis
Abstract
(R,S)-ketamine (ketamine) and its enantiomer (S)-ketamine (esketamine) can produce rapid and substantial antidepressant effects. However, individual response to ketamine/esketamine is variable, and there are no well-accepted methods to differentiate persons who are more likely to benefit. Numerous potential peripheral biomarkers have been reported, but their current utility is unclear. We conducted a systematic review/meta-analysis examining the association between baseline levels and longitudinal changes in blood-based biomarkers, and response to ketamine/esketamine. Of the 5611 citations identified, 56 manuscripts were included (N = 2801 participants), and 26 were compatible with meta-analytical calculations. Random-effect models were used, and effect sizes were reported as standardized mean differences (SMD). Our assessments revealed that more than 460 individual biomarkers were examined. Frequently studied groups included neurotrophic factors (n = 15), levels of ketamine and ketamine metabolites (n = 13), and inflammatory markers (n = 12). There were no consistent associations between baseline levels of blood-based biomarkers, and response to ketamine. However, in a longitudinal analysis, ketamine responders had statistically significant increases in brain-derived neurotrophic factor (BDNF) when compared to pre-treatment levels (SMD [95% CI] = 0.26 [0.03, 0.48], p = 0.02), whereas non-responders showed no significant changes in BDNF levels (SMD [95% CI] = 0.05 [-0.19, 0.28], p = 0.70). There was no consistent evidence to support any additional longitudinal biomarkers. Findings were inconclusive for esketamine due to the small number of studies (n = 2). Despite a diverse and substantial literature, there is limited evidence that blood-based biomarkers are associated with response to ketamine, and no current evidence of clinical utility.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTERESTS
TDG is listed as co-author on patent and patent applications related to the pharmacology and use of (
Figures
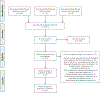
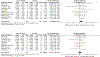

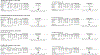
References
-
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. - PubMed
-
- Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168:689–701. - PubMed
-
- Thase ME, Mahableshwarkar AR, Dragheim M, Loft H, Vieta E. A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol. 2016;26:979–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

