An Argentinean cohort of patients with rheumatic and immune-mediated diseases vaccinated for SARS-CoV-2: the SAR-CoVAC Registry-protocol and preliminary data
- PMID: 35760939
- PMCID: PMC9244362
- DOI: 10.1007/s10067-022-06253-5
An Argentinean cohort of patients with rheumatic and immune-mediated diseases vaccinated for SARS-CoV-2: the SAR-CoVAC Registry-protocol and preliminary data
Abstract
Background/objective: To evaluate the efficacy and safety of SARS-CoV-2 vaccine in patients with rheumatic and immune-mediated inflammatory diseases (IMIDs) in Argentina: the SAR-CoVAC registry.
Methods: SAR-CoVAC is a national, multicenter, and observational registry. Adult patients with rheumatic or IMIDs vaccinated for SARS-CoV-2 were consecutively included between June 1 and September 17, 2021. Sociodemographic data, comorbidities, underlying rheumatic or IMIDs, treatments received, their modification prior to vaccination, and history of SARS-CoV-2 infection were recorded. In addition, date and place of vaccination, type of vaccine applied, scheme, adverse events (AE), disease flares, and new immune-mediated manifestations related to the vaccine were analyzed.
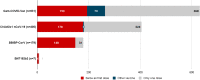
Results: A total of 1234 patients were included, 79% were female, with a mean age of 57.8 (SD 14.1) years. The most frequent diseases were rheumatoid arthritis (41.2%), osteoarthritis (14.5%), psoriasis (12.7%), and spondyloarthritis (12.3%). Most of them were in remission (28.5%) or low disease activity (41.4%). At the time of vaccination, 21% were receiving glucocorticoid treatment, 35.7% methotrexate, 29.7% biological (b) disease modifying anti-rheumatic drugs (DMARD), and 5.4% JAK inhibitors. In total, 16.9% had SARS-CoV-2 infection before the first vaccine dose. Most patients (51.1%) received Gam-COVID-Vac as the first vaccine dose, followed by ChAdOx1 nCoV-19 (32.8%) and BBIBP-CorV (14.5%). Half of them (48.8%) were fully vaccinated with 2 doses; 12.5% received combined schemes, being the most frequent Gam-COVID-Vac/mRAN-1273. The median time between doses was 51 days (IQR 53). After the first dose, 25.9% of the patients reported at least one AE and 15.9% after the second, being flu-like syndrome and local hypersensitivity the most frequent manifestations. There was one case of anaphylaxis. Regarding efficacy, 63 events of SARS-CoV-2 infection were reported after vaccination, 19% occurred during the first 14 days post-vaccination, 57.1% after the first dose, and 23.8% after the second. Most cases (85.9%) were asymptomatic or mild and 2 died due to COVID-19.
Conclusions: In this national cohort of patients, the most common vaccines used were Gam-COVID-Vac and ChAdOx1 nCoV-19. A quarter of the patients presented an AE and 5.1% presented SARS-CoV-2 infection after vaccination, in most cases mild.
Study registration: This study has been registered in ClinicalTrials.gov under the number: NCT04845997. Key Points • This study shows real-world data about efficacy and safety of SARS-CoV-2 vaccination in patients with rheumatic and immune-mediated inflammatory diseases. Interestingly, different types of vaccines were used including vector-based, mRNA, and inactivated vaccines, and mixed regimens were enabled. • A quarter of the patients presented an adverse event. The incidence of adverse events was significantly higher in those receiving mRAN-1273 and ChAdOx1 nCoV-19. • In this cohort, 5.1% presented SARS-CoV-2 infection after vaccination, in most cases mild.
Keywords: Argentina; COVID-19; Rheumatic diseases; SARS-CoV-2; Vaccines.
© 2022. The Author(s), under exclusive licence to International League of Associations for Rheumatology (ILAR).
Figures
References
-
- World Health Organization (2021) Cronología de la respuesta de la OMS a la COVID-19. https://www.who.int/es/news/item/29-06-2020-covidtimeline. Accessed 14 Febr 2021
-
- Ministerio de Salud. Argentina (2021) Comenzó la campaña de vacunación contra COVID-19 en Argentina. https://www.argentina.gob.ar/noticias/comenzo-la-campana-de-vacunacion-c.... Accessed 20 Feb 2021
-
- Ministerio de Salud. Argentina (2021) Plan Estratégico para la vacunación contra la COVID-19 en Argentina. https://www.argentina.gob.ar/sites/default/files/coronavirus-vacuna-plan.... Accessed 20 Feb 2021
-
- Ministerio de Salud. Argentina (2021) ¿Cuántas personas fueron vacunadas hasta ahora? https://www.argentina.gob.ar/coronavirus/vacuna. Accessed 25 Oct 2021
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous