Sulfated Glycoprotein Synthesis
- PMID: 35761038
- PMCID: PMC9721108
- DOI: 10.1007/978-1-0716-2489-0_1
Sulfated Glycoprotein Synthesis
Abstract
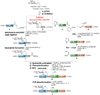
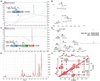
Chemical protein synthesis has achieved tremendous progress in the past decades. With the development of chemical ligation as powerful tools, the scope of synthetic protein is greatly expanded. Proteoglycans are a class of sulfated glycoproteins widely distributed on the cell surface and in the extracellular matrix, which are extensively engaged in cellular communication events. Consisting of protein backbone and glycosaminoglycan(s) side chain, proteoglycans are highly complex and heterogeneous in nature. Chemical synthesis provides facile and reliable approach to these molecules, with defined glycan structure and sulfation pattern. One remaining problem is that the acid-labile sulfates could hardly survive during the typical solid phase peptide synthesis (SPPS) process. In this chapter, strategic design of a "glycopeptide cassette" for the preparation of sulfated glycoprotein is described. In particular, we provide protocols for the chemical synthesis of ectodomain fragment (23-120) of sulfated glycoprotein syndecan-1.
Keywords: Glycopeptide cassette; Heparan sulfates; Protein chemical ligation; Proteoglycan; Sulfated glycoprotein.
© 2022. The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature.
Figures



References
-
- Kent SBH (2009) Total chemical synthesis of proteins. Chem Soc Rev 38:338–351 - PubMed
-
- Murakami M, Okamoto R, Izumi M, Kajihara Y (2012) Chemical synthesis of an erythropoietin Glycoform containing a complex-type Disialyloligosaccharide. Angew Chem Int Ed 51:3567–3572 - PubMed
-
- Lee CL, Liu H, Wong CTT, Chow HY, Li X (2016) Enabling N-to-C Ser/Thr ligation for convergent protein synthesis via combining chemical ligation approaches. J Am Chem Soc 138:10477–10484 - PubMed
-
- Li H, Zhang J, An C, Dong S (2021) Probing N-glycan functions in human interleukin-17A based on chemically synthesized homogeneous Glycoforms. J Am Chem Soc 143:2846–2856 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

