A tale of two transmitters: serotonin and histamine as in vivo biomarkers of chronic stress in mice
- PMID: 35761344
- PMCID: PMC9235270
- DOI: 10.1186/s12974-022-02508-9
A tale of two transmitters: serotonin and histamine as in vivo biomarkers of chronic stress in mice
Abstract
Background: Stress-induced mental illnesses (mediated by neuroinflammation) pose one of the world's most urgent public health challenges. A reliable in vivo chemical biomarker of stress would significantly improve the clinical communities' diagnostic and therapeutic approaches to illnesses, such as depression.
Methods: Male and female C57BL/6J mice underwent a chronic stress paradigm. We paired innovative in vivo serotonin and histamine voltammetric measurement technologies, behavioral testing, and cutting-edge mathematical methods to correlate chemistry to stress and behavior.
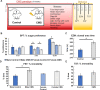
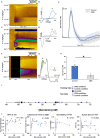
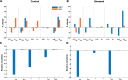
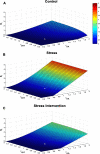
Results: Inflammation-induced increases in hypothalamic histamine were co-measured with decreased in vivo extracellular hippocampal serotonin in mice that underwent a chronic stress paradigm, regardless of behavioral phenotype. In animals with depression phenotypes, correlations were found between serotonin and the extent of behavioral indices of depression. We created a high accuracy algorithm that could predict whether animals had been exposed to stress or not based solely on the serotonin measurement. We next developed a model of serotonin and histamine modulation, which predicted that stress-induced neuroinflammation increases histaminergic activity, serving to inhibit serotonin. Finally, we created a mathematical index of stress, Si and predicted that during chronic stress, where Si is high, simultaneously increasing serotonin and decreasing histamine is the most effective chemical strategy to restoring serotonin to pre-stress levels. When we pursued this idea pharmacologically, our experiments were nearly identical to the model's predictions.
Conclusions: This work shines the light on two biomarkers of chronic stress, histamine and serotonin, and implies that both may be important in our future investigations of the pathology and treatment of inflammation-induced depression.
Keywords: Biomarkers; Depression; Histamine; Inflammation; Serotonin; Stress.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Depression Fact Sheet. https://www.who.int/news-room/fact-sheets/detail/depression.
-
- James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J, Abdelalim A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. - DOI - PMC - PubMed
-
- Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, Rasoulpoor S, Khaledi-Paveh B. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Glob Health. 2020;16:1–11. doi: 10.1186/s12992-019-0531-5. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01MH106563/Foundation for the National Institutes of Health
- R21MH109959/Foundation for the National Institutes of Health
- BB/N013956/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/N019008/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 200790/Z/16/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources

