CO2 Separation by Imide/Imine Organic Cages
- PMID: 35762229
- PMCID: PMC9545214
- DOI: 10.1002/chem.202201631
CO2 Separation by Imide/Imine Organic Cages
Abstract
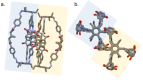
Two novel imide/imine-based organic cages have been prepared and studied as materials for the selective separation of CO2 from N2 and CH4 under vacuum swing adsorption conditions. Gas adsorption on the new compounds showed selectivity for CO2 over N2 and CH4 . The cages were also tested as fillers in mixed-matrix membranes for gas separation. Dense and robust membranes were obtained by loading the cages in either Matrimid® or PEEK-WC polymers. Improved gas-transport properties and selectivity for CO2 were achieved compared to the neat polymer membranes.
Keywords: carbon capture; gas separation; mixed-matrix membranes; organic cages; porous materials.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
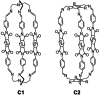
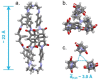
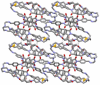
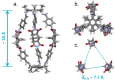

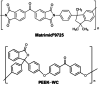
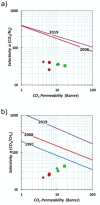
 : Matrimid® 9725/C1,
: Matrimid® 9725/C1,  : PEEK‐WC/C1.
: PEEK‐WC/C1.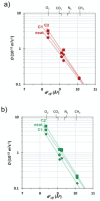
References
-
- Terlouw T., Treyer K., Bauer C., Mazzotti M., Environ. Sci. Technol. 2021, 55, 11397–11411. - PubMed
-
- Wu X., Yu Y., Qin Z., Zhang Z., Energy Prog. 2014, 63, 1339–1346.
-
- Chawla M., Saulat H., Masood Khan M., Mahmood Khan M., Rafiq S., Cheng L., Iqbal T., Rasheed M. I., Farooq M. Z., Saeed M., et al., Chem. Eng. Technol. 2020, 43, 184–199.
-
- Kanehashi S., Scholes C. A., Front. Chem. 2020, 14, 460–469.
-
- Dechnik J., Gascon J., Doonan C. J., Janiak C., Sumby C. J., Angew. Chem. Int. Ed. 2017, 56, 9292–9310; - PubMed
- Angew. Chem. 2017, 129, 9420–9439.
Grants and funding
LinkOut - more resources
Full Text Sources

