TGF-β1 peptide-based inhibitor P144 ameliorates renal fibrosis after ischemia-reperfusion injury by modulating alternatively activated macrophages
- PMID: 35762283
- PMCID: PMC9528764
- DOI: 10.1111/cpr.13299
TGF-β1 peptide-based inhibitor P144 ameliorates renal fibrosis after ischemia-reperfusion injury by modulating alternatively activated macrophages
Abstract
Objectives: Ischemia-reperfusion injury (IRI) is a major cause of chronic renal fibrosis. Currently, numerous therapies have shown a minimal effect on the blockade of fibrosis progression. Here, the therapeutic potential of peptide-based TGF-β1 inhibitor P144 in IRI-induced renal fibrosis and the underlying mechanism were analyzed.
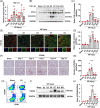
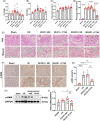
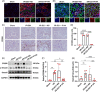
Materials and methods: The unilateral ischemia-reperfusion injury with the contralateral nephrectomy model was established, and the P144 was administered intravenously 1d/14d after the onset of IRI. The histopathology and immunofluorescence staining were used to detect renal fibrosis and macrophage infiltration. The in vivo fluorescence imaging was used to measure the bio-distribution of P144. The transwell assays were used to observe the migration of macrophages. RT-qPCR and western blot were used to analyze TGF-β1 signaling.
Results: P144 ameliorated the accumulation of extracellular matrix in the kidney and improved the renal function in the unilateral ischemia-reperfusion injury plus contralateral nephrectomy model. Mechanistically, P144 downregulated the TGF-β1-Smad3 signaling at both the transcriptional and translational levels and further reduced the TGF-β1-dependent infiltration of macrophages to the injured kidney. Additionally, P144 blocked the polarization of macrophages to an M2-like phenotype induced by TGF-β1 in vitro, but showed no effect on their proliferation.
Conclusions: Our study showed that the TGF-β1 peptide-based inhibitor P144 decreased renal fibrosis through the blockade of the TGF-β1-Smad3 signaling pathway and the modulation of macrophage polarization, suggesting its potential therapeutic use in IRI-induced renal fibrosis.
© 2022 The Authors. Cell Proliferation published by European Cell Proliferation Society and John Wiley & Sons Ltd.
Conflict of interest statement
The author declares that there are no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

