Empagliflozin for Heart Failure With Preserved Left Ventricular Ejection Fraction With and Without Diabetes
- PMID: 35762322
- PMCID: PMC9422757
- DOI: 10.1161/CIRCULATIONAHA.122.059785
Empagliflozin for Heart Failure With Preserved Left Ventricular Ejection Fraction With and Without Diabetes
Abstract
Background: Empagliflozin improves outcomes in patients with heart failure with a preserved ejection fraction, but whether the effects are consistent in patients with and without diabetes remains to be elucidated.
Methods: Patients with class II through IV heart failure and a left ventricular ejection fraction >40% were randomized to receive empagliflozin 10 mg or placebo in addition to usual therapy. We undertook a prespecified analysis comparing the effects of empagliflozin versus placebo in patients with and without diabetes.
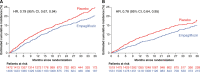
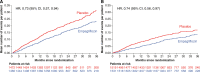
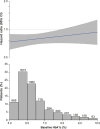
Results: Of the 5988 patients enrolled, 2938 (49%) had diabetes. The risk of the primary outcome (first hospitalization for heart failure or cardiovascular death), total hospitalizations for heart failure, and estimated glomerular filtration rate decline was higher in patients with diabetes. Empagliflozin reduced the rate of the primary outcome irrespective of diabetes status (hazard ratio, 0.79 [95% CI, 0.67, 0.94] for patients with diabetes versus hazard ratio, 0.78 [95% CI, 0.64, 0.95] in patients without diabetes; Pinteraction=0.92). The effect of empagliflozin to reduce total hospitalizations for heart failure was also consistent in patients with and without diabetes. The effect of empagliflozin to attenuate estimated glomerular filtration rate decline during double-blind treatment was also present in patients with and without diabetes, although more pronounced in patients with diabetes (1.77 in diabetes versus 0.98 mL/min/1.73m2 in patients without diabetes; Pinteraction=0.01). Across these 3 end points, the effect of empagliflozin did not differ in patients with prediabetes or normoglycemia (33% and 18% of the patient population, respectively). When investigated as a continuous variable, baseline hemoglobin A1c did not modify the effects on the primary outcome (Pinteraction=0.26). There was no increased risk of hypoglycemic events in either subgroup as compared with placebo.
Conclusions: In patients with heart failure and a preserved ejection fraction enrolled in the EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction), empagliflozin significantly reduced the risk of heart failure outcomes irrespective of diabetes status at baseline.
Registration: URL: https://www.
Clinicaltrials: gov; Unique identifier: NCT03057951.
Keywords: death; diabetes mellitus; heart failure; hospitalization; prognosis.
Figures


Comment in
-
In HFpEF, the benefit of empagliflozin on a composite of CV death or HF hospitalization at 26 mo did not vary by diabetes status.Ann Intern Med. 2022 Oct;175(10):JC111. doi: 10.7326/J22-0075. Epub 2022 Oct 4. Ann Intern Med. 2022. PMID: 36191324
References
-
- Seferovic PM, Petrie MC, Filippatos GS, Anker SD, Rosano G, Bauersachs J, Paulus WJ, Komajda M, Cosentino F, de Boer RA, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:853–872. doi: 10.1002/ejhf.1170 - PubMed
-
- McHugh K, DeVore AD, Wu J, Matsouaka RA, Fonarow GC, Heidenreich PA, Yancy CW, Green JB, Altman N, Hernandez AF. Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:602–611. doi: 10.1016/j.jacc.2018.11.033 - PubMed
-
- McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, Pratley R, Greenberg M, Wang S, Huyck S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes. JAMA Cardiol. 2021;6:1481–1411. doi: 10.1001/jamacardio.2020.4511 - PMC - PubMed
-
- Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. 2020;323:1353–1368. doi: 10.1001/jama.2020.1906 - PMC - PubMed
-
- Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

