A heterotypic assembly mechanism regulates CHIP E3 ligase activity
- PMID: 35762422
- PMCID: PMC9340540
- DOI: 10.15252/embj.2021109566
A heterotypic assembly mechanism regulates CHIP E3 ligase activity
Erratum in
-
Author Correction: A heterotypic assembly mechanism regulates CHIP E3 ligase activity.EMBO J. 2024 Mar;43(6):1110-1111. doi: 10.1038/s44318-024-00042-3. EMBO J. 2024. PMID: 38388749 Free PMC article.
Abstract
CHIP (C-terminus of Hsc70-interacting protein) and its worm ortholog CHN-1 are E3 ubiquitin ligases that link the chaperone system with the ubiquitin-proteasome system (UPS). CHN-1 can cooperate with UFD-2, another E3 ligase, to accelerate ubiquitin chain formation; however, the basis for the high processivity of this E3s set has remained obscure. Here, we studied the molecular mechanism and function of the CHN-1-UFD-2 complex in Caenorhabditis elegans. Our data show that UFD-2 binding promotes the cooperation between CHN-1 and ubiquitin-conjugating E2 enzymes by stabilizing the CHN-1 U-box dimer. However, HSP70/HSP-1 chaperone outcompetes UFD-2 for CHN-1 binding, thereby promoting a shift to the autoinhibited CHN-1 state by acting on a conserved residue in its U-box domain. The interaction with UFD-2 enables CHN-1 to efficiently ubiquitylate and regulate S-adenosylhomocysteinase (AHCY-1), a key enzyme in the S-adenosylmethionine (SAM) regeneration cycle, which is essential for SAM-dependent methylation. Our results define the molecular mechanism underlying the synergistic cooperation of CHN-1 and UFD-2 in substrate ubiquitylation.
Keywords: C. elegans; CHIP/STUB1/CHN-1; UFD-2; metabolism; ubiquitin ligase.
© 2022 The Authors. Published under the terms of the CC BY 4.0 license.
Figures

- A
Time‐dependent (0, 10, 20, 40, 80, and 120 min) CHN‐1 auto‐Ub was performed as indicated using UbWT and UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. Below, a graph representing the nmol of ubiquitylated CHN‐1 vs. time for CHN‐1 alone (black) or CHN‐1 + UFD‐2 (cyan). Plotted data are the mean of three technical replicates. Error bars represent the standard error of measurement (SEM); statistical significance was determined using Pearson's correlation coefficients which define the statistical relation between two continuous variables [CHN‐1 vs. time, CHN‐1 + UFD‐2 vs. time, and CHN‐1 vs. CHN‐1 + UFD‐2 with increasing time] (*P < 0.05; **P < 0.01; ***P < 0.001).
- B
CHN‐1 auto‐Ub was performed in the presence of UFD‐2 with the increasing molar concentration as indicated. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. Right, signal quantification of the unmodified CHN‐1 (yellow), ubiquitylated fraction < 70 kDa (cyan) and > 70 kDa (magenta), plotted as a percentage of different CHN‐1 species present in the indicated condition. Plotted data are the mean from the three technical replicates. Error bars represent the SEM; statistical significance was determined using a two‐way ANOVA test (**P < 0.01).
- C
Auto‐Ub was performed as indicated using recombinant CHN‐1 and UFD‐2P951A, UBE2D1 E2, UbWT, UbNoK, or Ub with substitutions of lysines 29, 48, and 63 to arginines (Ub3KTR). Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
- D
Surface plasmon resonance (SPR) sensorgrams of the interaction between linear di‐Ub (M1‐ linear from UbiQ) and C. elegans UFD‐2 (magenta) or S. cerevisiae Ufd2p (cyan). Y‐axis: Response unit (RU) value. X‐axis: nmolar (nM) concentration of linear di‐Ub.
- E
CHN‐1 auto‐Ub was performed as indicated in the presence of recombinant C. elegans UFD‐2 or S. cerevisiae Ufd2p and UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.

- A
Auto‐Ub of recombinant CHN‐1 only and in the presence of UFD‐2 was carried using UBE2D1, UBE2D2, or UBE2D3 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
- B
Auto‐Ub of recombinant UFD‐2 was performed using UBE2D1, UBE2D2, or UBE2D3 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐UFD‐2 antibodies.
- C
Auto‐Ub of recombinant CHN‐1 only and in the presence of UFD‐2 was carried using LET‐70 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
- D
In vitro ubiquitylation assay performed in the presence of CHN‐1, UFD‐2 or both as indicated using UBE2N‐Uev1a E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐Ub antibodies.
- E
Auto‐Ub of recombinant UFD‐2 only and in the presence of CHN‐1 was performed as indicated using UBE2N‐Uev1a, UBE2D1, or UBE2D3 E2, and UbWT or UbNoK. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐UFD‐2 antibodies.
- F
Auto‐Ub of recombinant CHN‐1H218Q was performed in the presence of UFD‐2 or UFD‐2P951A as indicated using UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. Asterisk (*) on the blot represented the signal that appeared in the presence of UFD‐2P951A.
- G
Time‐dependent (0, 30, 60, 120, and 180 min) auto‐Ub of CHN‐1 only and in the presence of UFD‐2 was performed as indicated using UBE2D2 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
- H
Time‐dependent (0, 30, 60, 120, and 180 min) auto‐Ub of CHN‐1 only and in the presence of UFD‐2 was performed as indicated using UBE2D3 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
- I
Auto‐Ub of recombinant CHN‐1Δ110 only and in the presence of UFD‐2 was performed as indicated using UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
- J
Co‐immunoprecipitation of ubiquitin‐charged GST‐UBE2D1 from a mixture of ubiquitin‐charged GST‐UBE2D1 and CHN‐1, ubiquitin‐charged GST‐UBE2D1 and UFD‐2P951A, or the ternary mixture of ubiquitin‐charged GST‐UBE2D1, CHN‐1 and UFD‐2P951A using Dynabeads conjugated with anti‐GST antibody. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐GST, anti‐UFD‐2, and anti‐CHN‐1 antibodies.
- K
Auto‐Ub of recombinant CHN‐1 only and in the presence of UFD‐2, UFD‐2P951A, or UFD‐2ΔUbox was performed as indicated using UBE2D1 E2. Bands were labeled as Unmodified CHN‐1, Multi‐mono‐Ubiquitylated CHN‐1, and Poly‐Ubiquitylated CHN‐1. Right, quantification of CHN‐1 modifications (Unmodified, Multi‐mono‐ubiquitylated, Poly‐ubiquitylated) plotted as percentages. Graph plotted for CHN‐1 alone (black), CHN‐1 + UFD‐2 (cyan), CHN‐1 + UFD‐2P951A (yellow), or CHN‐1 + UFD‐2ΔUbox (magenta). Plotted data are the mean of three technical replicates. Error bars represent the SEM; statistical significance was determined using a two‐way ANOVA test (****P < 0.0001).

- A
Size‐exclusion chromatography (SEC) profiles of the recombinant proteins CHN‐1 (cyan), UFD‐2 (magenta), and CHN‐1 + UFD‐2 mixture (black) resolved in the S200 Superdex column.
- B
Chiclet plot showing the differences in deuterium uptake by CHN‐1 peptides due to the presence of UFD‐2 across the five time points. The X‐axis spans the peptide length of CHN‐1 and the time points are plotted on the Y‐axis (total of 99 peptides with 84.2% sequence coverage and 4.55 redundancy). Above the chiclet plot is the domain organization of CHN‐1, indicating TPR and U‐box domains.
- C
Model of the CHN‐1 U‐box dimer with two E2 enzymes. UbcH5 E2 (gold) (PDB ID: 2OXQ) was aligned to the co‐crystal structure of CHIP (Danio rerio). The two structures aligned with low RMSD = 0.376. Marked are conserved residues that stabilize the critical interaction between the U‐box domain and E2.
- D
Discharging assay of Ub‐charged UBE2D1 was carried with increasing molar concentrations of recombinant UFD‐2P951A as indicated. The reaction was stopped after 30 min via the addition of Laemmli sample buffer. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐UBE2D1antibodies.
- E
CHN‐1 auto‐Ub was performed as indicated in the presence of Ube2W‐Ub or Ube2W‐UbFLAG with and without a complexing equimolar concentration of recombinant CHN‐1 and UFD‐2P951A and in the absence or presence of HSP‐1. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. For each sample, the quantified relative signal after probing the blot using anti‐CHN‐1 antibodies is represented as a ratio above the respective signal. Right, schematic of the CHN‐1‐UbFLAG (cyan), CHN‐1‐Ub (magenta), and unmodified CHN‐1 signal (black) presented as ratio and the signal fold change among CHN‐1, CHN‐1 + UFD‐2P951A, CHN‐1 + HSP‐1, and CHN‐1 + UFD‐2P951A + HSP‐1.

- A
HDX‐MS was used to analyze changes in the structural dynamics of residues within CHN‐1 when complexed with UFD‐2. The model diagram represents regions of retarded (red) and enhanced (blue) exchange in CHN‐1. Right, schematics showing CHN‐1 domain organization and the rate of deuterium exchange (colored box: blue, light red, medium red, and dark red) in the different domains upon interaction with UFD‐2.
- B
CHN‐1 auto‐Ub was performed as indicated using increasing molar concentrations (0.5, 1, 2, 3, 4 μM) of UBE2D1 E2 without and with a complexing equimolar concentration (1:1) of recombinant CHN‐1 and UFD‐2P951A (1.5 μM CHN‐1 with 1.5 μM of recombinant UFD‐2P951A). Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. Right, a graph representing the nmol of ubiquitylated CHN‐1 vs. UBE2D1 (μM) for CHN‐1 alone (black) or CHN‐1 + UFD‐2P951A (cyan). Plotted data are the mean from the three technical replicates. Error bars represent the SEM; statistical significance was determined using Pearson's correlation coefficients which define the statistical relation between two continuous variables [nmol of ubiquitylated CHN‐1 vs. UBE2D1(μM) in the presence of CHN‐1 or CHN‐1 + UFD‐2P951A and CHN‐1 vs. CHN‐1 + UFD‐2P951A with increasing UBE2D1(μM)] (*P < 0.05; ****P < 0.0001).
- C
Discharging assay of Ub‐charged UBE2D1 was carried out in the presence of CHN‐1 or CHN‐1‐UFD‐2P951A. The experimental sample was run together with the control with and without a reducing agent (50 mM DTT). The reaction was stopped after 30 min via the addition of Laemmli sample buffer. Proteins were resolved via SDS–PAGE and immunoblotted with anti‐UBE2D1 antibodies. Below, quantification of uncharged UBE2D1 plotted as the uncharged UBE2D1 fraction vs. UBE2D1‐Ub (μM). Plotted data are the mean of three technical replicates. Statistical significance was determined using Pearson's correlation coefficients which define the statistical relation between two continuous variables [UBE2D1 vs. UBE2D1‐Ub(μM) in the presence of CHN‐1 or CHN‐1 + UFD‐2P951A and CHN‐1 vs. CHN‐1 + UFD‐2P951A with increasing UBE2D1‐Ub(μM)] (P < 0.05; ***P < 0.001; ****P < 0.0001).
- D
CHN‐1 auto‐Ub was performed as indicated in the presence of Ube2W‐Ub or Ube2W‐UbFLAG without and with equimolar concentration of recombinant CHN‐1 and UFD‐2P951A. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. For each sample, the quantified relative signal after probing the blot using anti‐CHN‐1 antibodies is shown as a ratio above the respective signal. Below, a schematic of the CHN‐1‐UbFLAG (cyan), CHN‐1‐Ub (magenta), and unmodified CHN‐1 signal (black) presented as the ratio and the signal fold change between CHN‐1 and CHN‐1‐UFD‐2P951A.

- A
Auto‐Ub of recombinant CHN‐1 using UBE2D1 E2 was performed as indicated, alone or when complexed with UFD‐2, and in the presence of recombinant DAF‐21, DAF‐21ΔEEVD, HSP‐1, or HSP‐1ΔEEVD. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
- B
Top, schematics of the HSP‐1 peptide sequence (cyan) aligned with the C‐terminal sequence of full‐length HSP‐1 (amino acids 600–640) used in the ubiquitylation reaction. Auto‐Ub of recombinant CHN‐1 was performed as indicated in the presence of HSP‐1‐derived peptide and UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. Right, quantification of the CHN‐1 modifications (Unmodified, Multi‐mono‐ubiquitylated, Poly‐ubiquitylated) when CHN‐1 alone (black) or CHN‐1 + HSP‐1 peptide (cyan). Plotted data are the mean of three technical replicates. Error bars represent the SEM; statistical significance was determined using a two‐way ANOVA test (****P < 0.0001).
- C
Top, schematics of the UFD‐2 peptide sequences aligned with the C‐terminal sequence of full‐length UFD‐2 (amino acids 864–914) for PEPTIDE 1 (yellow) and PEPTIDE 2 (cyan), and (UFD‐2 amino acids 648–678) for PEPTIDE 3 (magenta) used in the ubiquitylation reaction. Below, auto‐Ub of recombinant CHN‐1 was performed as indicated in the presence of UFD‐2‐derived peptides using UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. Right, quantification of the CHN‐1 modifications (Unmodified, Multi‐mono‐ubiquitylated, Poly‐ubiquitylated) when CHN‐1 alone (black), CHN‐1 + PEPTIDE 1 (yellow), CHN‐1 + PEPTIDE 2 (cyan), or CHN‐1 + PEPTIDE 3 (magenta). Plotted data are the mean of three technical replicates. Error bars represent the SEM; statistical significance was determined using a two‐way ANOVA test (***P < 0.001).
- D
Auto‐Ub of recombinant CHN‐1 was performed as indicated using UBE2D1 E2 in the presence of recombinant HSP‐1 or HSP‐1EEYD. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
- E
Auto‐Ub of recombinant CHN‐1 was performed as indicated using UBE2D1 E2 in the presence of recombinant UFD‐2 or UFD‐2EEVD. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
- F
CHN‐1R230A auto‐Ub was performed as indicated using UBE2D1 E2 in the presence of recombinant UFD‐2 and HSP‐1. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
- G
Model of UFD‐2 activation and HSP‐1 inhibition of CHN‐1. Dimeric CHN‐1 with the TPR domain, U‐box, and helix‐turn‐helix (HH) is indicated by magenta, gold, and cyan, respectively. UFD‐2 and HSP‐1 peptides are shown in yellow with the indicated amino acid positions in the full‐length proteins.

- A
ELISA‐based titration assay to determine the dissociation constants (KD) between DAF‐21, HSP‐1, UFD‐2, and CHN‐1. Y‐axis: CHN‐1 concentration (μM). X‐axis: absorbance (OD) value at 450 nm as a function of the converted substrate (Alkaline Phosphatase Yellow). Below, a table showing the KD value (nM) of the corresponding protein with recombinant CHN‐1. Plotted data are the mean of three technical replicates. Error bars represent the SEM.
- B
ELISA‐based titration assay performed using recombinant CHN‐1, UFD‐2, and DAF‐21 with the results plotted as the DAF‐21 concentration (μM) vs. absorbance (OD) value at 450 nm as a function of the converted substrate (Alkaline Phosphatase Yellow). Plotted data are the mean of three technical replicates. Error bars represent the SEM.
- C
ELISA‐based titration assay performed using recombinant CHN‐1, UFD‐2, and HSP‐1 with the results plotted as the HSP‐1 concentration (μM) vs. absorbance (OD) value at 450 nm as a function of the converted substrate (Alkaline Phosphatase Yellow). Plotted data are the mean of three technical replicates. Error bars represent the SEM.
- D
In vitro ubiquitylation assay was performed as indicated using Ub‐charged UBE2D1 in the presence of CHN‐1, UFD‐2P951A or ternary mixture of recombinant CHN‐1, UFD‐2P951A and HSP‐1. The reaction was stopped after the indicated time via the addition of Laemmli sample buffer. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐Ubiquitin antibodies.
- E
Multiple sequence alignment (MSA) of UFD‐2 from different species. Orthologous sequences from selected species were obtained from the eggNOG5 database (from Orthologous Group ID ENOG5038DSP) (Huerta‐Cepas et al, 2019) and aligned using the T‐Coffee web server with default parameters (Notredame et al, ; Di Tommaso et al, 2011). Vertebrates possess two UFD‐2 orthologs, which have been independently annotated. The MSA was visualized in the Jalview Desktop software (Waterhouse et al, 2009) with residues colored according to their physicochemical properties; conserved tyrosine (Y) residues and the EEYD motif in C. elegans are highlighted in white frames.
- F
In vitro ubiquitylation assay was performed as indicated using an increasing concentration of Ub‐charged UBE2D1 (1.6, 3.3, 5, 6.6 μM) in the presence of CHN‐1 or CHN‐1 and HSP‐1EEYD. The reaction was stopped after 30 min via the addition of Laemmli sample buffer. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐Ubiquitin antibodies.
- G
Auto‐Ub of recombinant CHN‐1Δ87 (cyan) or CHN‐1Δ95 (magenta) truncation mutants as indicated using UBE2D1 E2 in the presence of UFD‐2, DAF‐21, DAF‐21ΔEEVD, HSP‐1 or HSP‐1ΔEEVD. Samples were analyzed via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies. Cyan asterisk (*) on the blot represented the auto‐ub of CHN‐1Δ87 and magenta asterisk (*) on the blot represented the auto‐ub of CHN‐1Δ95.
- H
Model of the CHN‐1 TPR domain docked with the UFD‐2 EEYD peptide. Residues 1–86 are colored in orange and residues 87–95 of CHN‐1, which sequester the EEYD motif away from the CHN‐1 R230 residue, are colored in magenta.
- I
A co‐crystal structure of the murine CHIP TPR domain interacting with the HSP90 EEVD peptide (PDB ID 2C2L) reveals that CHIP R273 (conserved in CHN‐1 as R230) is sufficiently close in proximity to interact with HSP90 D501.
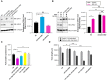
- A
To detect CHN‐1 and UFD‐2, indicated lysates of young adult worms were subjected to immunoblotting with anti‐CHN‐1 and anti‐UFD‐2 antibodies. Tubulin served as a loading control. Right, quantification of the CHN‐1 signals normalized to tubulin levels and plotted as N2 (wild‐type; black), ufd‐2(tm1380) (cyan), and UFD‐2P951A (magenta). Plotted data are the mean of three biological replicates. Error bars represent the SEM; statistical significance was determined using an unpaired t‐test (*P < 0.05).
- B
CHN‐1 protein levels were determined in the indicated lysates of young adult worms treated with a proteasome inhibitor (MG‐132, 10 μM) and DUB inhibitor (NEM, 100 mM). Tubulin served as a loading control. Right, quantification of the CHN‐1 signals normalized to tubulin levels plotted as control (black), or MG‐132 treated (magenta) in N2 (wild‐type) and ufd‐2(tm1380). Plotted data are the mean of three biological replicates. Error bars represent the SEM; statistical significance was determined using an unpaired t‐test (**P < 0.01; ***P < 0.001).
- C
Graph showing the comparison of protein solubility in the presence of 10% trichloroacetic acid (TCA) presented as TCA sensitive proteome in percentage (%) in N2 (wild‐type; black), ufd‐2(tm1380) (magenta), chn‐1(by155); ufd‐2(tm1380) (cyan), chn‐1(by155) (yellow) and UFD‐2P951A (gray) worms. Y‐axis shows the percent of the entire protein sample sensitive to TCA treatment. Plotted data are the mean of three biological replicates. Error bars represent SEM; statistical significance was determined using a one‐way ANOVA test (*P < 0.05; **P < 0.01).
- D
Measurement of the mobility of the indicated young adult worms exposed to 33°C heat stress (2 h). Graph plotted as control (gray), or recovery after heat stress (black) for N2 (wild‐type), ufd‐2(tm1380), chn‐1(by155); ufd‐2(tm1380), chn‐1(by155) and UFD‐2P951A worms. Plotted data are the mean of three biological replicates. Error bars represent SEM; statistical significance was determined using a one‐way ANOVA test (**P < 0.01; ****P < 0.0001).
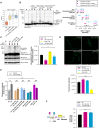
- A
Boxplot analysis showing the Z‐score of normalized intensities of the 50 LC‐MS/MS‐identified peptides from ACHY‐1 detected in N2 (wild‐type), chn‐1(by155), ufd‐2(tm1380), and chn‐1(by155); ufd‐2(tm1380) mutant worms. The central band of each box is the median value, and the box defines the 25th (lower) and 75th (higher) quantile. The whiskers represent the minimum and maximum values in the data, excluding outliers. A data point is considered an outlier if the distance to the median is greater than 1.5 * inter quantile range distance to the median.
- B
Ubiquitylation of recombinant AHCY‐1 was performed as indicated. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐AHCY‐1 antibodies. Right, quantification of the AHCY‐1 modifications (unmodified, oligo‐monoubiquitylated, poly‐ubiquitylated) when CHN‐1 alone (black), CHN‐1–UFD‐2 (magenta), or CHN‐1–UFD‐2EEVD (cyan). Plotted data are the mean of three technical replicates. Error bars represent SEM; statistical significance was determined using a two‐way ANOVA test (*P < 0.05).
- C
Protein level of endogenous AHCY‐1 in N2 (wild‐type), chn‐1(by155), CHN‐1 OE, and ufd‐2(tm1380) young adult worms treated with the proteasome inhibitor (MG‐132, 10 μM) and DUB inhibitor (NEM, 100 mM). Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐AHCY‐1 antibodies. Tubulin served as a loading control. Right, quantification of the modified AHCY‐1 signals plotted as Ub‐modified AHCY‐1 species normalized to unmodified endogenous AHCY‐1 signal and plotted for N2 (wild‐type; black), chn‐1(by155) (magenta), CHN‐1 OE (yellow), and ufd‐2(tm1380) (cyan). Plotted data are the mean of three biological replicates. Error bars represent SEM; statistical significance was determined using an unpaired t‐test (**P < 0.01).
- D
Representative images of GFP::AHCY‐1 fluorescence in chn‐1(by155), ufd‐2(tm1380), and CHN‐1 OE background. Scale bar = 200 μm. Below, quantification of the AHCY‐1 GFP signal plotted as fluorescence intensity for GFP::AHCY‐1 expressing worms (control; black), chn‐1(by155) (magenta), ufd‐2(tm1380) (cyan) or CHN‐1 OE (yellow). Plotted data are the mean of three biological replicates. Error bars represent SEM; statistical significance was determined using a one‐way ANOVA test (****P < 0.0001).
- E
Total lipid content in N2 (wild‐type), chn‐1(by155), ufd‐2(tm1380), chn‐1(by155), ufd‐2(tm1380), and CHN‐1 OE young adult worms grown on control (plain) and ahcy‐1(RNAi) (lined) feeding plates. Higher fluorescence intensity indicates increased lipid levels. Plotted data are the mean of three biological replicates. Error bars indicate SEM; statistical significance was determined using a one‐way ANOVA test (***P < 0.001).
- F
Schematic diagram representing the core function of AHCY‐1. AHCY‐1 catalyzes the reversible hydrolysis of SAH (S‐adenosylhomocysteine) to HCy (homocysteine). SAH accumulation inhibits PC (phosphatidylcholine) synthesis from PE (phosphatidylethanolamine). Right, ratio of phosphatidylcholine (PC) to phosphatidylethanolamine (PE) in N2 (wild‐type; black), chn‐1(by155) (magenta), and ufd‐2(tm1380) (cyan) young adult worms. Plotted data are the mean of three biological replicates. Error bars indicate SEM; statistical significance was determined using a one‐way ANOVA test (***P < 0.001).

- A
PCA showing the first and second principal components of the significantly altered proteins (ANOVA FDR < 0.05) performed in the Perseus software (Tyanova et al, 2016). The percentage of explained variance is represented on the axis labels.
- B
Schematic representation of the number of identified proteins in a single‐shot analysis of LC‐MS/MS gradients in five biological replicates that led to the identification of proteins with significant abundance changes in chn‐1(by155), ufd‐2(tm1380), and chn‐1(by155); ufd‐2(tm1380) worms (twofold enrichment in all mutants versus wild‐type N2 animals).
- C
Hierarchical clustering of the Z‐scores of proteins whose levels increased in chn‐1(by155), ufd‐2(tm1380), and chn‐1(by155); ufd‐2(tm1380) mutant worms (twofold enrichment in all mutants versus wild‐type N2 animals from the LC‐MS/MS experiment).
- D
Gene ontology biological process terms found to be associated with C. elegans genes upregulated (minimum twofold enrichment versus N2 (control), with FDR < 0.05 for ANOVA or pairwise t‐test) in all mutants; all proteins detected in the LC‐MS/MS analysis comprised a reference set. Overrepresentation analysis was performed using the WebGestalt web server with default parameters (Liao et al, 2019). FDR was controlled to 0.25 using the Benjamini‐Hochberg method for multiple testing.
- E
Yeast two‐hybrid prey fragment analysis. Schematic representations of the AHCY‐1 fragments interacting with CHN‐1 identified in the yeast two‐hybrid screen (Hybrigenics). The coding sequence for CHN‐1 was used as bait to screen a random‐primed C. elegans mixed‐stage cDNA library. The selected interaction domain (SID) is shown in yellow.
- F
Co‐immunoprecipitation of AHCY‐1 from young adult worms expressing CHN‐1::FLAG using beads conjugated with anti‐FLAG antibody. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐AHCY‐1 and anti‐FLAG antibodies (the red boxes mark the protein band).
- G
CHN‐1 auto‐Ub was performed as indicated. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐CHN‐1 antibodies.
- H
Ubiquitylation of recombinant AHCY‐1 was performed as indicated using recombinant UFD‐2 and UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐AHCY‐1 antibodies.
- I
Ubiquitylation of recombinant AHCY‐1 was performed as indicated using recombinant CHN‐1, UFD‐2, DAF‐21, or HSP‐1 in the presence of UBE2D1 E2. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐AHCY‐1 antibodies.
- J
Quantitative PCR analyses of ahcy‐1 transcript levels in young adult N2 (wild‐type; black), chn‐1(by155) (magenta) and ufd‐2(tm1380) (cyan) worms. Plotted data are the mean of three biological replicates. Error bars represent SEM; statistical significance was determined using a one‐way ANOVA test.
- K
Protein level of endogenous AHCY‐1 in N2 (wild‐type), chn‐1(by155), and ufd‐2(tm1380) young adult worms. After centrifugation, the supernatant obtained from the worm lysate and the resulting pellet were dissolved in 5% SDS and boiled for 5 min. Protein samples were resolved via SDS–PAGE and immunoblotted with anti‐AHCY‐1 antibodies.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

