Ca2+ attenuates nucleation activity of leiomodin
- PMID: 35762710
- PMCID: PMC9207750
- DOI: 10.1002/pro.4358
Ca2+ attenuates nucleation activity of leiomodin
Abstract
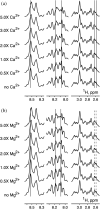
A transient increase in Ca2+ concentration in sarcomeres is essential for their proper function. Ca2+ drives striated muscle contraction via binding to the troponin complex of the thin filament to activate its interaction with the myosin thick filament. In addition to the troponin complex, the myosin essential light chain and myosin-binding protein C were also found to be Ca2+ sensitive. However, the effects of Ca2+ on the function of the tropomodulin family proteins involved in regulating thin filament formation have not yet been studied. Leiomodin, a member of the tropomodulin family, is an actin nucleator and thin filament elongator. Using pyrene-actin polymerization assay and transmission electron microscopy, we show that the actin nucleation activity of leiomodin is attenuated by Ca2+ . Using circular dichroism and nuclear magnetic resonance spectroscopy, we demonstrate that the mostly disordered, negatively charged region of leiomodin located between its first two actin-binding sites binds Ca2+ . We propose that Ca2+ binding to leiomodin results in the attenuation of its nucleation activity. Our data provide further evidence regarding the role of Ca2+ as an ultimate regulator of the ensemble of sarcomeric proteins essential for muscle function. SUMMARY STATEMENT: Ca2+ fluctuations in striated muscle sarcomeres modulate contractile activity via binding to several distinct families of sarcomeric proteins. The effects of Ca2+ on the activity of leiomodin-an actin nucleator and thin filament length regulator-have remained unknown. In this study, we demonstrate that Ca2+ binds directly to leiomodin and attenuates its actin nucleating activity. Our data emphasizes the ultimate role of Ca2+ in the regulation of the sarcomeric protein interactions.
Keywords: actin; circular dichroism; leiomodin; nuclear magnetic resonance; pyrene-actin polymerization; transmission electron microscopy.
© 2022 The Authors. Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Figures




References
-
- DuVall MM, Gifford JL, Amrein M, Herzog W. Altered mechanical properties of titin immunoglobulin domain 27 in the presence of calcium. Eur Biophys J. 2013;42:301–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

