An Activatable 19 F MRI Molecular Probe for Sensing and Imaging of Norepinephrine
- PMID: 35762743
- PMCID: PMC9278097
- DOI: 10.1002/open.202200110
An Activatable 19 F MRI Molecular Probe for Sensing and Imaging of Norepinephrine
Abstract
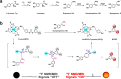
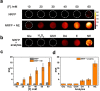
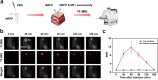
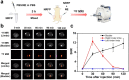
Norepinephrine (NE), acting as both a neurotransmitter and hormone, plays a significant role in regulating the action of the brain and body. Many studies have demonstrated a strong correlation between mental disorders and aberrant NE levels. Therefore, it is of urgent demand to develop in vivo analytical methods of NE for diagnostic assessment and mechanistic investigations of mental diseases. Herein, we report a 19 F MRI probe (NRFP) for sensing and imaging NE, which is constructed by conjugating a gadolinium chelate to a fluorine-containing moiety through a NE-responsive aromatic thiocarbonate linkage. The capacity and specificity of NRFP for detecting NE is validated with in vitro detecting/imaging experiments. Furthermore, the feasibility of NRFP for visualizing NE in animals is illustrated by ex vivo and in vivo imaging experiments, demonstrating the promising potential of NRFP for selective detection and specific imaging of NE in deep tissues of living subjects.
Keywords: 19F NMR/MRI; deep-tissue sensing; imaging agents; in vivo imaging; norepinephrine.
© 2022 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Novel 19F activatable probe for the detection of matrix metalloprotease-2 activity by MRI/MRS.Mol Pharm. 2014 Nov 3;11(11):4208-17. doi: 10.1021/mp500443x. Epub 2014 Oct 13. Mol Pharm. 2014. PMID: 25271556 Free PMC article.
-
Recent Research Progress of 19 F Magnetic Resonance Imaging Probes: Principle, Design, and Their Application.Macromol Rapid Commun. 2023 Aug;44(16):e2200744. doi: 10.1002/marc.202200744. Epub 2023 Jan 5. Macromol Rapid Commun. 2023. PMID: 36512446 Review.
-
Activatable 19F MRI nanoparticle probes for the detection of reducing environments.Angew Chem Int Ed Engl. 2015 Jan 12;54(3):1007-10. doi: 10.1002/anie.201409365. Epub 2014 Nov 20. Angew Chem Int Ed Engl. 2015. PMID: 25413833
-
A fluorinated bihydrazide conjugate for activatable sensing and imaging of hypochlorous acid by 19F NMR/MRI.Chem Commun (Camb). 2019 Oct 15;55(83):12455-12458. doi: 10.1039/c9cc06622e. Chem Commun (Camb). 2019. PMID: 31565704
-
Fluorine polymer probes for magnetic resonance imaging: quo vadis?MAGMA. 2019 Feb;32(1):173-185. doi: 10.1007/s10334-018-0724-6. Epub 2018 Nov 29. MAGMA. 2019. PMID: 30498886 Free PMC article. Review.
Cited by
-
Novel non‑metal‑based contrast agents for MR imaging: Emerging approaches and clinical perspectives (Review).Int J Oncol. 2025 Aug;67(2):70. doi: 10.3892/ijo.2025.5776. Epub 2025 Jul 19. Int J Oncol. 2025. PMID: 40682851 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

