Innovations in the prevention and treatment of postpartum hemorrhage: Analysis of a novel medicines development pipeline database
- PMID: 35762804
- PMCID: PMC9328148
- DOI: 10.1002/ijgo.14200
Innovations in the prevention and treatment of postpartum hemorrhage: Analysis of a novel medicines development pipeline database
Abstract
Background: A significant barrier to improving prevention and treatment of postpartum hemorrhage (PPH) is a lack of innovative medicines that meet the needs of women and providers, particularly those in low-and middle-income countries (LMICs). The Accelerating Innovation for Mothers (AIM) project established a new database of candidate medicines under development for five pregnancy-related conditions between 2000 and 2021.
Objective: To systematically identify and rank candidates for prevention and treatment of PPH.
Search strategy: Adis Insight, Pharmaprojects, WHO ICTRP, PubMed, and grant databases were searched to develop the AIM database.
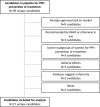
Selection criteria: AIM database was searched for candidates being evaluated for PPH prevention and treatment, regardless of phase.
Data collection and analysis: Candidates were ranked as high, medium, or low potential based on prespecified criteria. Analysis was primarily descriptive, describing candidates and development potential.
Main results: Of the 444 unique candidates, only 39 pertained to PPH. One was high potential (heat-stable/inhaled oxytocin) and three were medium potential (melatonin, vasopressin and dofetilide via nanoparticle delivery).
Conclusion: The pipeline for new PPH medicines is concerningly limited, lacking diversity, and showing little evidence of novel technologies. Without significant investment in early-phase research, it is unlikely that new products will emerge.
Keywords: innovation; medicines; postpartum hemorrhage; prevention; treatment.
© 2022 The Authors. International Journal of Gynecology & Obstetrics published by John Wiley & Sons Ltd on behalf of International Federation of Gynecology and Obstetrics.
Conflict of interest statement
AMG and AA are Concept Foundation staff who provide regulatory assistance to the developers of heat‐stable carbetocin and inhaled oxytocin. SR reports funding to Concept Foundation from MSD for Mothers. AA, AMcD, AMG, AT, JPV, and MG report grant funding to Concept Foundation from the Bill & Melinda Gates Foundation (Investment ID INV‐023749). Outside the present study, A. M. G. reports an honorarium from Ferring Pharmaceuticals paid to Concept Foundation for PPH panel participation (International Confederation of Midwives Conference, June 23, 2021). Other authors have no conflicts of interest to declare.
Figures

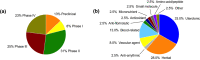
References
-
- Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol. 2008;22:999‐1012. - PubMed
-
- World Health Organization . WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. WHO; 2012. - PubMed
-
- Naz H, Sarwar I, Fawad A, Nisa AU. Maternal morbidity and mortality due to primary PPH‐‐experience at Ayub teaching hospital Abbottabad. J Ayub Med Coll Abbottabad. 2008;20:59‐65. - PubMed
-
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323‐e333. - PubMed
-
- World Health Organization, UNICEF, UNFPA, World Bank Group, United Nations Population Division . Trends in Maternal Mortality 2000 to 2017: Estimates by WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. WHO; 2019.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

