Opportunities and Challenges When Using the Electronic Health Record for Practice-Integrated Patient-Facing Interventions: The e-Assist Colon Health Randomized Trial
- PMID: 35762832
- PMCID: PMC9583291
- DOI: 10.1177/0272989X221104094
Opportunities and Challenges When Using the Electronic Health Record for Practice-Integrated Patient-Facing Interventions: The e-Assist Colon Health Randomized Trial
Abstract
Background: Even after a physician recommendation, many people remain unscreened for colorectal cancer (CRC). The proliferation of electronic health records (EHRs) and tethered online portals may afford new opportunities to embed patient-facing interventions within clinic workflows and engage patients following a physician recommendation for care. We evaluated the effectiveness of a patient-facing intervention designed to complement physician office-based recommendations for CRC screening.
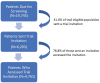
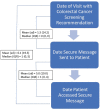
Design: Using a 2-arm pragmatic, randomized clinical trial, we evaluated the intervention's effect on CRC screening use as documented in the EHR (primary outcome) and the extent to which the intervention reached the target population. Trial participants were insured, aged 50 to 75 y, with a physician recommendation for CRC screening. Typical EHR functionalities, including patient registries, health maintenance flags, best practice alerts, and secure messaging, were used to support research-related activities and deliver the intervention to enrolled patients.
Results: A total of 1,825 adults consented to trial participation, of whom 78% completed a baseline survey and were exposed to the intervention. Most trial participants (>80%) indicated an intent to be screened on the baseline survey, and 65% were screened at follow-up, with no significant differences by study arm. One-third of eligible patients were sent a secure message. Among those, more than three-quarters accessed study material.
Conclusions: By leveraging common EHR functionalities, we integrated a patient-facing intervention within clinic workflows. Despite practice integration, the intervention did not improve screening use, likely in part due to portal-based interventions not reaching those for whom the intervention may be most effective.
Implications: Embedding patient-facing interventions within the EHR enabled practice integration but may minimize program effectiveness by missing important segments of the patient population.
Highlights: Electronic health record tools can be used to facilitate practice-embedded pragmatic trial and patient-facing intervention processes, including patient identification, study arm allocation, and intervention delivery.The online portal-embedded intervention did not improve colorectal cancer (CRC) screening uptake following a physician recommendation, likely in part because portal users tend to be already highly engaged with healthcare.Relying on patient portals alone for CRC screening interventions may not alter screening use and could exacerbate well-known care disparities.
Keywords: colorectal cancer screening; hierarchical generalized linear models; patient portals; program reach.
Figures
References
-
- American Cancer Society. Colorectal Cancer Facts and Figures 2020-2022. Atlanta (GA): American Cancer Society; 2020.
-
- Cairns CP, Viswanath K. Communication and colorectal cancer screening among the uninsured: data from the Health Information National Trends Survey (United States). Cancer Causes & Control. 2006;17(9):1115–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical