PET imaging of hepatocellular carcinoma by targeting tumor-associated endothelium using [68Ga]Ga-PSMA-617
- PMID: 35763056
- PMCID: PMC9529836
- DOI: 10.1007/s00259-022-05884-9
PET imaging of hepatocellular carcinoma by targeting tumor-associated endothelium using [68Ga]Ga-PSMA-617
Abstract
Objective: Hepatocellular carcinoma (HCC) is a malignant tumor associated with high morbidity and mortality rates. In many non-prostate solid tumors such as HCC, prostate-specific membrane antigens (PSMA) are overexpressed in tumor-associated endothelial cells. Therefore, the aim of this study was to evaluate the performance of [68Ga]Ga-PSMA-617 PET imaging on HCC with different animal models, including cell line-derived xenografts (CDX) and patient-derived xenografts (PDX), and to explore its mechanisms of function.
Methods: [68Ga]Ga-PSMA-617 was prepared. The expression level of PSMA in two human hepatocellular cancer cells (HepG2 and HuH-7) was evaluated, and the cellular uptakes of [68Ga]Ga-PSMA-617 were assayed. HepG2 and HuH-7 subcutaneous xenograft models, HepG2 orthotopic xenograft models, and four different groups of PDX models were prepared. Preclinical pharmacokinetics and performance of [68Ga]Ga-PSMA-617 were evaluated in different types of HCC xenografts models using small animal PET and biodistribution studies.
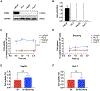
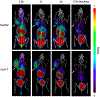
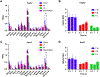
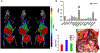
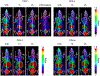
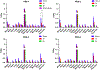
Results: Low PSMA expression level of HepG2 and HuH-7 cells was observed, and the cellular uptake and blocking study confirmed the non-specificity of the PSMA-targeted probe binding to HepG2 and HuH-7 cells. In the subcutaneous xenograft models, the tumor uptakes at 0.5 h were 0.76 ± 0.12%ID/g (HepG2 tumors) and 0.78 ± 0.08%ID/g (HuH-7 tumors), respectively, which were significantly higher than those of the blocking groups (0.23 ± 0.04%ID/g and 0.20 ± 0.04%ID/g, respectively). In the orthotopic xenograft models, PET images clearly displayed the tumor locations based on the preferential accumulation of [68Ga]Ga-PSMA-617 in tumor tissue versus normal liver tissue, suggesting the possibility of using [68Ga]Ga-PSMA-617 PET imaging to detect primary HCC lesions in deep tissue. In the four different groups of HCC PDX models, PET imaging with [68Ga]Ga-PSMA-617 provided clear tumor uptakes with prominent tumor-to-background contrast, further demonstrating its potential for the clinical imaging of PSMA-positive HCC lesions. The staining of tumor tissue sections with CD31- and PSMA-specific antibodies visualized the tumor-associated blood vessels and PSMA expression on endothelial cells in subcutaneous, orthotopic tissues, and PDX tissues, confirming the imaging with [68Ga]Ga-PSMA-617 might be mediated by targeting tumor associated endothelium.
Conclusion: In this study, in vivo PET on different types of HCC xenograft models illustrated high uptake within tumors, which confirmed that [68Ga]Ga-PSMA-617 PET may be a promising imaging modality for HCC by targeting tumor associated endothelium.
Keywords: Diagnosis; Hepatocellular carcinoma (HCC); Positron emission tomography (PET); Prostate-specific membrane antigen (PSMA); Tumor-associated endothelial cells.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Figures


Comment in
-
PSMA-targeted theranostics of solid tumors: applications beyond prostate cancers.Eur J Nucl Med Mol Imaging. 2022 Oct;49(12):3973-3976. doi: 10.1007/s00259-022-05905-7. Eur J Nucl Med Mol Imaging. 2022. PMID: 35916921 No abstract available.
-
The era of prostate-specific membrane antigen (PSMA)-based theranostics for hepatocellular carcinoma is upcoming: are we ready for it?Eur J Nucl Med Mol Imaging. 2022 Oct;49(12):3977-3978. doi: 10.1007/s00259-022-05928-0. Epub 2022 Aug 10. Eur J Nucl Med Mol Imaging. 2022. PMID: 35947176 No abstract available.
-
Letter to the editor regarding "PET imaging of hepatocellular carcinoma by targeting tumor‑associated endothelium using [68 Ga]Ga‑PSMA‑617".Eur J Nucl Med Mol Imaging. 2023 Feb;50(3):638. doi: 10.1007/s00259-022-06005-2. Epub 2022 Oct 17. Eur J Nucl Med Mol Imaging. 2023. PMID: 36251024 No abstract available.
-
Validation and utility of HepG2 xenograft model for hepatocellular carcinoma.Eur J Nucl Med Mol Imaging. 2023 Feb;50(3):639-641. doi: 10.1007/s00259-022-06043-w. Epub 2022 Nov 22. Eur J Nucl Med Mol Imaging. 2023. PMID: 36416907 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

